Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins
- PMID: 23552419
- PMCID: PMC3676108
- DOI: 10.1128/JVI.00635-13
Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins
Abstract
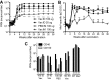
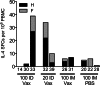
DNA vaccines formulated with the cationic lipid-based adjuvant Vaxfectin induce protective immunity in macaques after intradermal (i.d.) or intramuscular (i.m.) delivery of 0.5 to 1 mg of codon-optimized DNA encoding the hemagglutinin (H) and fusion (F) proteins of measles virus (MeV). To characterize the effect of Vaxfectin at lower doses of H+F DNA, rhesus macaques were vaccinated twice with 20 μg of DNA plus Vaxfectin i.d., 100 μg of DNA plus Vaxfectin i.d., 100 μg of DNA plus Vaxfectin i.m. or 100 μg of DNA plus phosphate-buffered saline (PBS) i.m. using a needleless Biojector device. The levels of neutralizing (P = 0.036) and binding (P = 0.0001) antibodies were higher after 20 or 100 μg of DNA plus Vaxfectin than after 100 μg of DNA plus PBS. Gamma interferon (IFN-γ)-producing T cells were induced more rapidly than antibody, but were not improved with Vaxfectin. At 18 months after vaccination, monkeys were challenged with wild-type MeV. None developed rash or viremia, but all showed evidence of infection. Antibody levels increased, and IFN-γ- and interleukin-17-producing T cells, including cells specific for the nucleoprotein absent from the vaccine, were induced. At 3 months after challenge, MeV RNA was detected in the leukocytes of two monkeys. The levels of antibody peaked 2 to 4 weeks after challenge and then declined in vaccinated animals reflecting low numbers of bone marrow-resident plasma cells. Therefore, Vaxfectin was dose sparing and substantially improved the antibody response to the H+F DNA vaccine. This immune response led to protection from disease (rash/viremia) but not from infection. Antibody responses after challenge were more transient in vaccinated animals than in an unvaccinated animal.
Figures




Similar articles
-
Use of Vaxfectin adjuvant with DNA vaccine encoding the measles virus hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques against measles virus.Clin Vaccine Immunol. 2008 Aug;15(8):1214-21. doi: 10.1128/CVI.00120-08. Epub 2008 Jun 4. Clin Vaccine Immunol. 2008. PMID: 18524884 Free PMC article.
-
Poor immune responses of newborn rhesus macaques to measles virus DNA vaccines expressing the hemagglutinin and fusion glycoproteins.Clin Vaccine Immunol. 2013 Feb;20(2):205-10. doi: 10.1128/CVI.00394-12. Epub 2012 Dec 12. Clin Vaccine Immunol. 2013. PMID: 23239799 Free PMC article.
-
A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles.J Virol. 2010 Apr;84(8):3798-807. doi: 10.1128/JVI.01566-09. Epub 2010 Feb 3. J Virol. 2010. PMID: 20130066 Free PMC article.
-
Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles.Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11581-8. doi: 10.1073/pnas.0504592102. Epub 2005 Jul 21. Proc Natl Acad Sci U S A. 2005. PMID: 16037211 Free PMC article.
-
Measles Vaccine.Viral Immunol. 2018 Mar;31(2):86-95. doi: 10.1089/vim.2017.0143. Epub 2017 Dec 19. Viral Immunol. 2018. PMID: 29256824 Free PMC article. Review.
Cited by
-
Immunogenicity after outbreak response immunization activities among young healthcare workers with secondary vaccine failure during the measles epidemic in Korea, 2019.BMC Infect Dis. 2022 Jun 8;22(1):530. doi: 10.1186/s12879-022-07511-2. BMC Infect Dis. 2022. PMID: 35676650 Free PMC article.
-
The Immune Response in Measles: Virus Control, Clearance and Protective Immunity.Viruses. 2016 Oct 12;8(10):282. doi: 10.3390/v8100282. Viruses. 2016. PMID: 27754341 Free PMC article. Review.
-
Measles Virus Neutralizing Antibody Response, Cell-Mediated Immunity, and Immunoglobulin G Antibody Avidity Before and After Receipt of a Third Dose of Measles, Mumps, and Rubella Vaccine in Young Adults.J Infect Dis. 2016 Apr 1;213(7):1115-23. doi: 10.1093/infdis/jiv555. Epub 2015 Nov 23. J Infect Dis. 2016. PMID: 26597262 Free PMC article.
-
A Recombinant Measles Vaccine with Enhanced Resistance to Passive Immunity.Viruses. 2017 Sep 21;9(10):265. doi: 10.3390/v9100265. Viruses. 2017. PMID: 28934110 Free PMC article.
-
Generation of a More Immunogenic Measles Vaccine by Increasing Its Hemagglutinin Expression.J Virol. 2016 May 12;90(11):5270-5279. doi: 10.1128/JVI.00348-16. Print 2016 Jun 1. J Virol. 2016. PMID: 26984727 Free PMC article.
References
-
- Nandy R, Handzel T, Zaneidou M, Biey J, Coddy RZ, Perry R, Strebel P, Cairns L. 2006. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clin. Infect. Dis. 42:322–328 - PubMed
-
- Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. 2009. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int. J. Epidemiol. 38:192–205 - PubMed
-
- Center for Disease Control 2007. Progress in global measles control and mortality reduction, 2000–2006. MMWR Morb. Mortal. Wkly. Rep. 56:1237–1242 - PubMed
-
- Moss WJ, Griffin DE. 2012. Measles. Lancet 379:153–164 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

