Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures
- PMID: 23552422
- PMCID: PMC3676115
- DOI: 10.1128/JVI.00009-13
Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures
Erratum in
-
Erratum for Chan et al., "Tropism of and Innate Immune Responses to the Novel Human Betacoronavirus Lineage C Virus in Human Ex Vivo Respiratory Organ Cultures".J Virol. 2024 Apr 16;98(4):e0037924. doi: 10.1128/jvi.00379-24. Epub 2024 Mar 18. J Virol. 2024. PMID: 38497665 Free PMC article. No abstract available.
Abstract
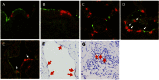
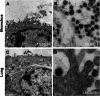
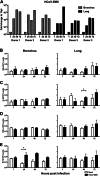
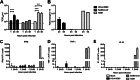
Since April 2012, there have been 17 laboratory-confirmed human cases of respiratory disease associated with newly recognized human betacoronavirus lineage C virus EMC (HCoV-EMC), and 7 of them were fatal. The transmissibility and pathogenesis of HCoV-EMC remain poorly understood, and elucidating its cellular tropism in human respiratory tissues will provide mechanistic insights into the key cellular targets for virus propagation and spread. We utilized ex vivo cultures of human bronchial and lung tissue specimens to investigate the tissue tropism and virus replication kinetics following experimental infection with HCoV-EMC compared with those following infection with human coronavirus 229E (HCoV-229E) and severe acute respiratory syndrome coronavirus (SARS-CoV). The innate immune responses elicited by HCoV-EMC were also investigated. HCoV-EMC productively replicated in human bronchial and lung ex vivo organ cultures. While SARS-CoV productively replicated in lung tissue, replication in human bronchial tissue was limited. Immunohistochemistry revealed that HCoV-EMC infected nonciliated bronchial epithelium, bronchiolar epithelial cells, alveolar epithelial cells, and endothelial cells. Transmission electron microscopy showed virions within the cytoplasm of bronchial epithelial cells and budding virions from alveolar epithelial cells (type II). In contrast, there was minimal HCoV-229E infection in these tissues. HCoV-EMC failed to elicit strong type I or III interferon (IFN) or proinflammatory innate immune responses in ex vivo respiratory tissue cultures. Treatment of human lung tissue ex vivo organ cultures with type I IFNs (alpha and beta IFNs) at 1 h postinfection reduced the replication of HCoV-EMC, suggesting a potential therapeutic use of IFNs for treatment of human infection.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

