The adenovirus L4-33K protein regulates both late gene expression patterns and viral DNA packaging
- PMID: 23552425
- PMCID: PMC3676125
- DOI: 10.1128/JVI.00652-13
The adenovirus L4-33K protein regulates both late gene expression patterns and viral DNA packaging
Abstract
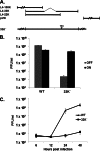
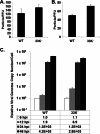
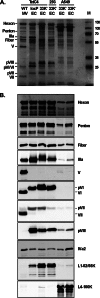
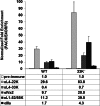
The adenovirus (Ad) L4-33K protein has been linked to disparate functions during infection. L4-33K is a virus-encoded alternative RNA splicing factor which activates splicing of viral late gene transcripts that contain weak 3' splice sites. Additionally, L4-33K has been indicated to play a role in adenovirus assembly. We generated and characterized an Ad5 L4-33K mutant virus to further explore its function(s) during infection. Infectivity, viral genome replication, and most viral gene expression of the L4-33K mutant virus are comparable to those of the wild-type virus, except for a prominent decrease in the levels of the late proteins IIIa and pVI. The L4-33K mutant virus produces only empty capsids, indicating a defect in viral DNA packaging. We demonstrate that L4-33K does not preferentially bind to viral packaging sequences in vivo, and mutation of L4-33K does not interfere with the binding of the known viral packaging proteins IVa2, L4-22K, L1-52/55K, and IIIa to the packaging sequences in vivo. Collectively, these results demonstrate that the phenotype of an Ad5 L4-33K mutant virus is complex. The L4-33K protein regulates the accumulation of selective Ad late gene mRNAs and is involved in the proper transition of gene expression during the late phase of infection. The L4-33K protein also plays a role in adenovirus morphogenesis by promoting the packaging of the viral genome into the empty capsid. These results demonstrate the multifunctional nature of the L4-33K protein and its involvement in several different and critical aspects of viral infection.
Figures


Similar articles
-
The adenovirus L4-22K protein has distinct functions in the posttranscriptional regulation of gene expression and encapsidation of the viral genome.J Virol. 2013 Jul;87(13):7688-99. doi: 10.1128/JVI.00859-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637408 Free PMC article.
-
The adenovirus L4-22K protein is multifunctional and is an integral component of crucial aspects of infection.J Virol. 2012 Oct;86(19):10474-83. doi: 10.1128/JVI.01463-12. Epub 2012 Jul 18. J Virol. 2012. PMID: 22811519 Free PMC article.
-
Serine 192 in the tiny RS repeat of the adenoviral L4-33K splicing enhancer protein is essential for function and reorganization of the protein to the periphery of viral replication centers.Virology. 2012 Nov 25;433(2):273-81. doi: 10.1016/j.virol.2012.08.021. Epub 2012 Sep 1. Virology. 2012. PMID: 22944109
-
Regulation of human adenovirus alternative RNA splicing by the adenoviral L4-33K and L4-22K proteins.Int J Mol Sci. 2015 Jan 28;16(2):2893-912. doi: 10.3390/ijms16022893. Int J Mol Sci. 2015. PMID: 25636034 Free PMC article. Review.
-
Mysteries of adenovirus packaging.J Virol. 2025 May 20;99(5):e0018025. doi: 10.1128/jvi.00180-25. Epub 2025 Apr 17. J Virol. 2025. PMID: 40243339 Free PMC article. Review.
Cited by
-
Porcine Adenovirus Type 3 E3 Encodes a Structural Protein Essential for Capsid Stability and Production of Infectious Progeny Virions.J Virol. 2018 Sep 26;92(20):e00680-18. doi: 10.1128/JVI.00680-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30068639 Free PMC article.
-
Adenovirus with DNA Packaging Gene Mutations Increased Virus Release.Viruses. 2016 Dec 20;8(12):333. doi: 10.3390/v8120333. Viruses. 2016. PMID: 27999391 Free PMC article.
-
Characterization of the function of Adenovirus L4 gene products and their impact on AAV vector production.Mol Ther Methods Clin Dev. 2024 Nov 4;32(4):101370. doi: 10.1016/j.omtm.2024.101370. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39640223 Free PMC article.
-
The adenovirus L4-22K protein has distinct functions in the posttranscriptional regulation of gene expression and encapsidation of the viral genome.J Virol. 2013 Jul;87(13):7688-99. doi: 10.1128/JVI.00859-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637408 Free PMC article.
-
Components of Adenovirus Genome Packaging.Front Microbiol. 2016 Sep 23;7:1503. doi: 10.3389/fmicb.2016.01503. eCollection 2016. Front Microbiol. 2016. PMID: 27721809 Free PMC article. Review.
References
-
- Berk AJ. 2005. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 24:7673–7685 - PubMed
-
- Akusjarvi G. 2008. Temporal regulation of adenovirus major late alternative RNA splicing. Front. Biosci. 13:5006–5015 - PubMed
-
- Larsson S, Svensson C, Akusjarvi G. 1992. Control of adenovirus major late gene expression at multiple levels. J. Mol. Biol. 225:287–298 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

