β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation
- PMID: 23552693
- PMCID: PMC3667720
- DOI: 10.1091/mbc.E12-12-0908
β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation
Abstract
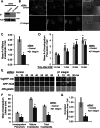
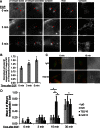
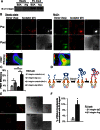
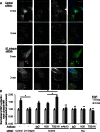
β1 integrin has been shown to promote metastasis in a number of tumor models, including breast, ovarian, pancreatic, and skin cancer; however, the mechanism by which it does so is poorly understood. Invasive membrane protrusions called invadopodia are believed to facilitate extracellular matrix degradation and intravasation during metastasis. Previous work showed that β1 integrin localizes to invadopodia, but its role in regulating invadopodial function has not been well characterized. We find that β1 integrin is required for the formation of mature, degradation-competent invadopodia in both two- and three-dimensional matrices but is dispensable for invadopodium precursor formation in metastatic human breast cancer cells. β1 integrin is activated during invadopodium precursor maturation, and forced β1 integrin activation enhances the rate of invadopodial matrix proteolysis. Furthermore, β1 integrin interacts with the tyrosine kinase Arg and stimulates Arg-dependent phosphorylation of cortactin on tyrosine 421. Silencing β1 integrin with small interfering RNA completely abrogates Arg-dependent cortactin phosphorylation and cofilin-dependent barbed-end formation at invadopodia, leading to a significant decrease in the number and stability of mature invadopodia. These results describe a fundamental role for β1 integrin in controlling actin polymerization-dependent invadopodial maturation and matrix degradation in metastatic tumor cells.
Figures



References
-
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. - PubMed
-
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal-antibody 9EG7 defines a novel beta(1) integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. - PubMed
-
- Bharadwaj S, Thanawala R, Bon G, Falcioni R, Prasad GL. Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene. 2005;24:8291–8303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

