Downregulation of miRNA-31 induces taxane resistance in ovarian cancer cells through increase of receptor tyrosine kinase MET
- PMID: 23552883
- PMCID: PMC3641356
- DOI: 10.1038/oncsis.2013.3
Downregulation of miRNA-31 induces taxane resistance in ovarian cancer cells through increase of receptor tyrosine kinase MET
Abstract
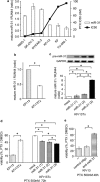
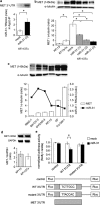
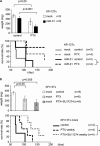
Ovarian cancer is one of the most aggressive female reproductive tract tumors. Paclitaxel (PTX) is widely used for the treatment of ovarian cancer. However, ovarian cancers often acquire chemotherapeutic resistance to this agent. We investigated the mechanism of chemoresistance by analysis of microRNAs using the ovarian cancer cell line KFr13 and its PTX-resistant derivative (KFr13Tx). We found that miR-31 was downregulated in KFr13Tx cells, and that re-introduction of miR31 re-sensitized them to PTX both in vitro and in vivo. miR-31 was found to bind to the 3'-UTR of mRNA of MET, and the decrease in MET correlated to higher sensitivity to PTX. Furthermore, co-treatment of KFr13Tx cells with MET inhibitors sensitized the tumor cells to PTX both in vitro and in vivo. In addition, lower levels of miR31 and higher expression of MET in human ovarian cancer specimens were significantly correlated with PTX chemoresistance and poor prognosis. This study demonstrated miR31-dependent regulation of MET for chemoresistance of ovarian cancer, raising the possibility that combination therapy with a MET inhibitor and PTX will increase PTX efficacy.
Figures


References
-
- Boyle P, Levin B.World cancer report 2008 World Health Organization, Lyon; 2008. 2008.
-
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. - PubMed
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. - PubMed
-
- Kristensen GB, Trope C. Epithelial ovarian carcinoma. Lancet. 1997;349:113–117. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

