Macromitophagy is a longevity assurance process that in chronologically aging yeast limited in calorie supply sustains functional mitochondria and maintains cellular lipid homeostasis
- PMID: 23553280
- PMCID: PMC3651518
- DOI: 10.18632/aging.100547
Macromitophagy is a longevity assurance process that in chronologically aging yeast limited in calorie supply sustains functional mitochondria and maintains cellular lipid homeostasis
Abstract
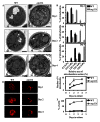
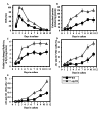
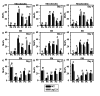
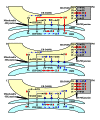
Macromitophagy controls mitochondrial quality and quantity. It involves the sequestration of dysfunctional or excessive mitochondria within double-membrane autophagosomes, which then fuse with the vacuole/lysosome to deliver these mitochondria for degradation. To investigate a physiological role of macromitophagy in yeast, we examined how theatg32Δ-dependent mutational block of this process influences the chronological lifespan of cells grown in a nutrient-rich medium containing low (0.2%) concentration of glucose. Under these longevity-extending conditions of caloric restriction (CR) yeast cells are not starving. We also assessed a role of macromitophagy in lifespan extension by lithocholic acid (LCA), a bile acid that prolongs yeast longevity under CR conditions. Our findings imply that macromitophagy is a longevity assurance process underlying the synergistic beneficial effects of CR and LCA on yeast lifespan. Our analysis of how the atg32Δ mutation influences mitochondrial morphology, composition and function revealed that macromitophagy is required to maintain a network of healthy mitochondria. Our comparative analysis of the membrane lipidomes of organelles purified from wild-type and atg32Δ cells revealed that macromitophagy is required for maintaining cellular lipid homeostasis. We concluded that macromitophagy defines yeast longevity by modulating vital cellular processes inside and outside of mitochondria.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to declare.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

