Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway
- PMID: 23553603
- PMCID: PMC4165612
- DOI: 10.1002/glia.22491
Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway
Abstract
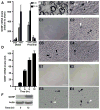
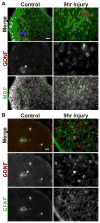
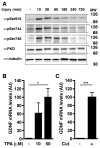
Upon peripheral nerve injury, specific molecular events, including increases in the expression of selected neurotrophic factors, are initiated to prepare the tissue for regeneration. However, the mechanisms underlying these events and the nature of the cells involved are poorly understood. We used the injury-induced upregulation of glial cell-derived neurotrophic factor (GDNF) expression as a tool to gain insights into these processes. We found that both myelinating and nonmyelinating Schwann cells are responsible for the dramatic increase in GDNF expression after injury. We also demonstrate that the GDNF upregulation is mediated by a signaling cascade involving activation of Schwann cell purinergic receptors, followed by protein kinase C signaling which activates protein kinase D (PKD), which leads to increased GDNF transcription. Given the potent effects of GDNF on survival and repair of injured peripheral neurons, we propose that targeting these pathways may yield therapeutic tools to treat peripheral nerve injury and neuropathies.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
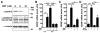
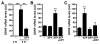
References
-
- Akkina SK, Patterson CL, Wright DE. GDNF rescues nonpeptidergic unmyelinated primary afferents in streptozotocin-treated diabetic mice. Exp Neurol. 2001;167(1):173–82. - PubMed
-
- Bampton ET, Taylor JS. Effects of Schwann cell secreted factors on PC12 cell neuritogenesis and survival. J Neurobiol. 2005;63(1):29–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

