The unique disulfide bond-stabilized W1 β4-β1 loop in the α4 β-propeller domain regulates integrin α4β7 affinity and signaling
- PMID: 23553626
- PMCID: PMC3656279
- DOI: 10.1074/jbc.M113.462630
The unique disulfide bond-stabilized W1 β4-β1 loop in the α4 β-propeller domain regulates integrin α4β7 affinity and signaling
Abstract
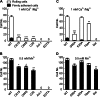
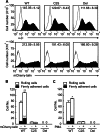
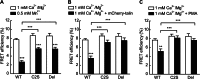
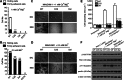
Integrin α4β7 mediates rolling and firm adhesion of lymphocytes pre- and post-activation, which is distinct from most integrins only mediating firm cell adhesion upon activation. This two-phase cell adhesion suggests a unique molecular basis for the dynamic interaction of α4β7 with its ligand, mucosal addressin cell adhesion molecule 1 (MAdCAM-1). Here we report that a disulfide bond-stabilized W1 β4-β1 loop in α4 β-propeller domain plays critical roles in regulating integrin α4β7 affinity and signaling. Either breaking the disulfide bond or deleting the disulfide bond-occluded segment in the W1 β4-β1 loop inhibited rolling cell adhesion supported by the low-affinity interaction between MAdCAM-1 and inactive α4β7 but negligibly affected firm cell adhesion supported by the high-affinity interaction between MAdCAM-1 and Mn(2+)-activated α4β7. Additionally, disrupting the disulfide bond or deleting the disulfide bond-occluded segment not only blocked the conformational change and activation of α4β7 triggered by talin or phorbol-12-myristate-13-acetate via inside-out signaling but also disrupted integrin-mediated outside-in signaling and impaired phosphorylation of focal adhesion kinase and paxillin. Thus, these findings reveal a particular molecular basis for α4β7-mediated rolling cell adhesion and a novel regulatory element of integrin affinity and signaling.
Keywords: Affinity Regulation; Cell Adhesion; Cell Signaling; Cell Surface Receptor; Integrin; Integrin α4β7; Ligand Binding Protein; W1 β4-β1 Loop.
Figures



References
-
- Hynes R. O. (2002) Integrins. Bidirectional, allosteric signaling machines. Cell 110, 673–687 - PubMed
-
- Berlin C., Bargatze R. F., Campbell J. J., von Andrian U. H., Szabo M. C., Hasslen S. R., Nelson R. D., Berg E. L., Erlandsen S. L., Butcher E. C. (1995) α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80, 413–422 - PubMed
-
- Bargatze R. F., Jutila M. A., Butcher E. C. (1995) Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ. The multistep model confirmed and refined. Immunity 3, 99–108 - PubMed
-
- Tözeren A., Kleinman H. K., Wu S., Mercurio A. M., Byers S. W. (1994) Integrin α 6 β 4 mediates dynamic interactions with laminin. J. Cell Sci. 107, 3153–3163 - PubMed
-
- Sigal A., Bleijs D. A., Grabovsky V., van Vliet S. J., Dwir O., Figdor C. G., van Kooyk Y., Alon R. (2000) The LFA-1 integrin supports rolling adhesions on ICAM-1 under physiological shear flow in a permissive cellular environment. J. Immunol. 165, 442–452 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

