Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine
- PMID: 23553628
- PMCID: PMC3656302
- DOI: 10.1074/jbc.M113.459222
Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine
Abstract
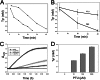
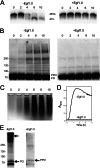
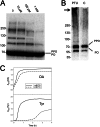
The phenoloxidase (PO) cascade regulates the melanization of blood (hemolymph) in insects and other arthropods. Most studies indicate that microbial elicitors activate the PO cascade, which results in processing of the zymogen PPO to PO. PO is then thought to oxidize tyrosine and o-diphenols to quinones, which leads to melanin. However, different lines of investigation raise questions as to whether these views are fully correct. Here we report that hemolymph from the silkmoth, Bombyx mori, rapidly melanizes after collection from a wound site. Prior studies indicated that in vitro activated PPO hydroxylates Tyr inefficiently. Measurement of in vivo substrate titers, however, suggested that Tyr was the only PO substrate initially present in B. mori plasma and that it is rapidly metabolized by PO. Fractionation of plasma by gel filtration chromatography followed by bioassays indicated that melanization activity was primarily associated with a high mass complex (~670 kDa) that contained PO. The prophenoloxidase-activating protease inhibitor Egf1.0 blocked formation of this complex and Tyr metabolism, but the addition of phenylthiourea to plasma before fractionation enhanced complex formation and Tyr metabolism. Mass spectrometry analysis indicated that the complex contained PO plus other proteins. Taken together, our results indicate that wounding alone activates the PO cascade in B. mori. They also suggest that complex formation is required for efficient use of Tyr as a substrate.
Keywords: Amino Acid; Blood; Cascade; Insect Immunity; Melanin; Phenoloxidase; Protease; Wound Healing.
Figures




Similar articles
-
Altered tyrosine metabolism and melanization complex formation underlie the developmental regulation of melanization in Manduca sexta.Insect Biochem Mol Biol. 2015 Mar;58:66-75. doi: 10.1016/j.ibmb.2015.01.002. Epub 2015 Jan 13. Insect Biochem Mol Biol. 2015. PMID: 25596090
-
Nosema bombycis suppresses host hemolymph melanization through secreted serpin 6 inhibiting the prophenoloxidase activation cascade.J Invertebr Pathol. 2019 Nov;168:107260. doi: 10.1016/j.jip.2019.107260. Epub 2019 Oct 14. J Invertebr Pathol. 2019. PMID: 31622597
-
The hemolymph melanization response is related to defence against the AcMNPV infection in Bombyx mori.Arch Insect Biochem Physiol. 2021 Sep;108(1):e21764. doi: 10.1002/arch.21764. Epub 2021 Jul 16. Arch Insect Biochem Physiol. 2021. PMID: 34272769
-
Innate Immunity in Insects: The Lights and Shadows of Phenoloxidase System Activation.Int J Mol Sci. 2025 Feb 4;26(3):1320. doi: 10.3390/ijms26031320. Int J Mol Sci. 2025. PMID: 39941087 Free PMC article. Review.
-
Role and importance of phenoloxidase in insect hemostasis.J Innate Immun. 2011;3(1):28-33. doi: 10.1159/000321931. Epub 2010 Nov 6. J Innate Immun. 2011. PMID: 21051882 Review.
Cited by
-
Iron-polyphenol complexes cause blackening upon grinding Hermetia illucens (black soldier fly) larvae.Sci Rep. 2019 Feb 27;9(1):2967. doi: 10.1038/s41598-019-38923-x. Sci Rep. 2019. PMID: 30814530 Free PMC article.
-
Efficiency of RNA interference is improved by knockdown of dsRNA nucleases in tephritid fruit flies.Open Biol. 2019 Dec;9(12):190198. doi: 10.1098/rsob.190198. Epub 2019 Dec 4. Open Biol. 2019. PMID: 31795920 Free PMC article.
-
Hemocyte Changes During Immune Melanization in Bombyx Mori Infected with Escherichia coli.Insects. 2019 Sep 16;10(9):301. doi: 10.3390/insects10090301. Insects. 2019. PMID: 31527493 Free PMC article.
-
Bombyx mori and Aedes aegypti form multi-functional immune complexes that integrate pattern recognition, melanization, coagulants, and hemocyte recruitment.PLoS One. 2017 Feb 15;12(2):e0171447. doi: 10.1371/journal.pone.0171447. eCollection 2017. PLoS One. 2017. PMID: 28199361 Free PMC article.
-
Analytical validation of a portable human Accu-Chek glucometer in honeybee hemolymph.J Vet Diagn Invest. 2022 Sep;34(5):789-795. doi: 10.1177/10406387221117233. Epub 2022 Aug 21. J Vet Diagn Invest. 2022. PMID: 35993265 Free PMC article.
References
-
- Cerenius L., Lee B. L., Söderhäll K. (2008) The proPO-system. Pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271 - PubMed
-
- Kanost M. R., Gorman M. J. (2008) Phenoloxidases in insect immunity. in Insect Immunology (Beckage N. E., ed) pp. 69–96, Academic Press, Inc., San Diego
-
- Nappi A., Poirié M., Carton Y. (2009) The role of melanization and cytotoxic by-products in the cellular immune responses of Drosophila against parasitic wasps. in Advances in Parasitology (Genevieve P., ed) pp. 99–121, Academic Press, Inc., San Diego - PubMed
-
- Sugumaran M. (2002) Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 15, 2–9 - PubMed
-
- Cerenius L., Söderhäll K. (2004) The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198, 116–126 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

