Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis
- PMID: 23553635
- PMCID: PMC3641226
- DOI: 10.1104/pp.113.215913
Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis
Abstract
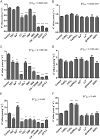
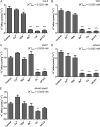
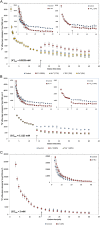
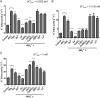
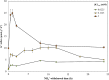
The role of potassium (K(+)) transporters in high- and low-affinity K(+) uptake was examined in roots of intact barley (Hordeum vulgare) and Arabidopsis (Arabidopsis thaliana) plants by use of (42)K radiotracing, electrophysiology, pharmacology, and mutant analysis. Comparisons were made between results from barley and five genotypes of Arabidopsis, including single and double knockout mutants for the high-affinity transporter, AtHAK5, and the Shaker-type channel, AtAKT1. In Arabidopsis, steady-state K(+) influx at low external K(+) concentration ([K(+)]ext = 22.5 µm) was predominantly mediated by AtAKT1 when high-affinity transport was inhibited by ammonium, whereas in barley, by contrast, K(+) channels could not operate below 100 µm. Withdrawal of ammonium resulted in an immediate and dramatic stimulation of K(+) influx in barley, indicating a shift from active to passive K(+) uptake at low [K(+)]ext and yielding fluxes as high as 36 µmol g (root fresh weight)(-1) h(-1) at 5 mm [K(+)]ext, among the highest transporter-mediated K(+) fluxes hitherto reported. This ammonium-withdrawal effect was also established in all Arabidopsis lines (the wild types, atakt1, athak5, and athak5 atakt1) at low [K(+)]ext, revealing the concerted involvement of several transport systems. The ammonium-withdrawal effect coincided with a suppression of K(+) efflux and a significant hyperpolarization of the plasma membrane in all genotypes except athak5 atakt1, could be sustained over 24 h, and resulted in increased tissue K(+) accumulation. We discuss key differences and similarities in K(+) acquisition between two important model systems and reveal novel aspects of K(+) transport in planta.
Figures


Similar articles
-
A Ca(2+)-sensitive system mediates low-affinity K(+) uptake in the absence of AKT1 in Arabidopsis plants.Plant Cell Physiol. 2012 Dec;53(12):2047-59. doi: 10.1093/pcp/pcs140. Epub 2012 Oct 10. Plant Cell Physiol. 2012. PMID: 23054389
-
Studies on Arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K uptake.Physiol Plant. 2010 Jun 1;139(2):220-8. doi: 10.1111/j.1399-3054.2010.01354.x. Epub 2010 Jan 19. Physiol Plant. 2010. PMID: 20088908
-
Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations.Physiol Plant. 2008 Dec;134(4):598-608. doi: 10.1111/j.1399-3054.2008.01168.x. Physiol Plant. 2008. PMID: 19000196
-
Root K(+) acquisition in plants: the Arabidopsis thaliana model.Plant Cell Physiol. 2011 Sep;52(9):1603-12. doi: 10.1093/pcp/pcr096. Epub 2011 Jul 19. Plant Cell Physiol. 2011. PMID: 21771865 Review.
-
K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms.J Plant Physiol. 2014 May 15;171(9):688-95. doi: 10.1016/j.jplph.2013.09.021. Epub 2014 Mar 3. J Plant Physiol. 2014. PMID: 24810767 Review.
Cited by
-
Comparison between Arabidopsis and Rice for Main Pathways of K(+) and Na(+) Uptake by Roots.Front Plant Sci. 2016 Jul 5;7:992. doi: 10.3389/fpls.2016.00992. eCollection 2016. Front Plant Sci. 2016. PMID: 27458473 Free PMC article. Review.
-
Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana.Int J Mol Sci. 2020 Dec 22;22(1):2. doi: 10.3390/ijms22010002. Int J Mol Sci. 2020. PMID: 33374906 Free PMC article.
-
The Role of Silicon in Higher Plants under Salinity and Drought Stress.Front Plant Sci. 2016 Jul 18;7:1072. doi: 10.3389/fpls.2016.01072. eCollection 2016. Front Plant Sci. 2016. PMID: 27486474 Free PMC article. Review.
-
Potassium in Root Growth and Development.Plants (Basel). 2019 Oct 22;8(10):435. doi: 10.3390/plants8100435. Plants (Basel). 2019. PMID: 31652570 Free PMC article. Review.
-
The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels.Plant Physiol. 2014 Oct;166(2):945-59. doi: 10.1104/pp.114.246520. Epub 2014 Aug 25. Plant Physiol. 2014. PMID: 25157029 Free PMC article.
References
-
- Alemán F, Nieves-Cordones M, Martínez V, Rubio F. (2011) Root K(+) acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol 52: 1603–1612 - PubMed
-
- Amrutha RN, Sekhar PN, Varshney RK, Kishor PBK. (2007) Genome-wide analysis and identification of genes related to potassium transporter families in rice (Oryza sativa L.). Plant Sci 172: 708–721
-
- Ashley MK, Grant M, Grabov A. (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57: 425–436 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

