Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals
- PMID: 23553643
- PMCID: PMC3933734
- DOI: 10.1002/hep.26431
Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals
Abstract
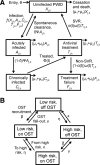
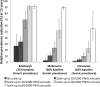
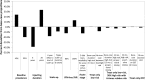
Substantial reductions in hepatitis C virus (HCV) prevalence among people who inject drugs (PWID) cannot be achieved by harm reduction interventions such as needle exchange and opiate substitution therapy (OST) alone. Current HCV treatment is arduous and uptake is low, but new highly effective and tolerable interferon-free direct-acting antiviral (DAA) treatments could facilitate increased uptake. We projected the potential impact of DAA treatments on PWID HCV prevalence in three settings. A dynamic HCV transmission model was parameterized to three chronic HCV prevalence settings: Edinburgh, UK (25%); Melbourne, Australia (50%); and Vancouver, Canada (65%). Using realistic scenarios of future DAAs (90% sustained viral response, 12 weeks duration, available 2015), we projected the treatment rates required to reduce chronic HCV prevalence by half or three-quarters within 15 years. Current HCV treatment rates may have a minimal impact on prevalence in Melbourne and Vancouver (<2% relative reductions) but could reduce prevalence by 26% in 15 years in Edinburgh. Prevalence could halve within 15 years with treatment scale-up to 15, 40, or 76 per 1,000 PWID annually in Edinburgh, Melbourne, or Vancouver, respectively (2-, 13-, and 15-fold increases, respectively). Scale-up to 22, 54, or 98 per 1,000 PWID annually could reduce prevalence by three-quarters within 15 years. Less impact occurs with delayed scale-up, higher baseline prevalence, or shorter average injecting duration. Results are insensitive to risk heterogeneity or restricting treatment to PWID on OST. At existing HCV drug costs, halving chronic prevalence would require annual treatment budgets of US $3.2 million in Edinburgh and approximately $50 million in Melbourne and Vancouver.
Conclusion: Interferon-free DAAs could enable increased HCV treatment uptake among PWID, which could have a major preventative impact. However, treatment costs may limit scale-up, and should be addressed.
© 2013 The Authors. Hepatology published by Wiley on behalf of the American Association for the Study of Liver Diseases.
Figures


Comment in
-
Treatment as prevention: the breaking of taboos is required in the fight against hepatitis C among people who inject drugs.Hepatology. 2013 Nov;58(5):1523-5. doi: 10.1002/hep.26539. Epub 2013 Sep 17. Hepatology. 2013. PMID: 23728921 No abstract available.
References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infec Dis. 2005;5:558–567. - PubMed
-
- Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Int Med. 2012;156:271–278. - PubMed
-
- Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure? Aust New Zealand J Pub Health. 2007;31:30–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G1000021/MRC_/Medical Research Council/United Kingdom
- R01DA011591/DA/NIDA NIH HHS/United States
- PDF-2011-04-049/DH_/Department of Health/United Kingdom
- R03 DA033851/DA/NIDA NIH HHS/United States
- RP-DG-0610-10055/DH_/Department of Health/United Kingdom
- R01 DA011591/DA/NIDA NIH HHS/United States
- WT087640MA/WT_/Wellcome Trust/United Kingdom
- CRUK_/Cancer Research UK/United Kingdom
- BHF_/British Heart Foundation/United Kingdom
- 129971-1/CAPMC/ CIHR/Canada
- G0701627/MRC_/Medical Research Council/United Kingdom
- MR/J013560/1/MRC_/Medical Research Council/United Kingdom
- G0801627/MRC_/Medical Research Council/United Kingdom
- 125948-1/CAPMC/ CIHR/Canada
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
