NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM
- PMID: 23553831
- PMCID: PMC3631659
- DOI: 10.1073/pnas.1303932110
NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM
Abstract
Small-cell lung cancer and other aggressive neuroendocrine cancers are often associated with early dissemination and frequent metastases. We demonstrate that neurogenic differentiation 1 (NeuroD1) is a regulatory hub securing cross talk among survival and migratory-inducing signaling pathways in neuroendocrine lung carcinomas. We find that NeuroD1 promotes tumor cell survival and metastasis in aggressive neuroendocrine lung tumors through regulation of the receptor tyrosine kinase tropomyosin-related kinase B (TrkB). Like TrkB, the prometastatic signaling molecule neural cell adhesion molecule (NCAM) is a downstream target of NeuroD1, whose impaired expression mirrors loss of NeuroD1. TrkB and NCAM may be therapeutic targets for aggressive neuroendocrine cancers that express NeuroD1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

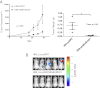

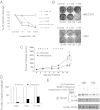
References
-
- Fischer B, Arcaro A. Current status of clinical trials for small cell lung cancer. Rev Recent Clin Trials. 2008;3(1):40–61. - PubMed
-
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366(9494):1385–1396. - PubMed
-
- Sato M, Shames DS, Gazdar AF, Minna JD. A translational view of the molecular pathogenesis of lung cancer. J Thorac Oncol. 2007;2(4):327–343. - PubMed
-
- Radice PA, et al. The clinical behavior of “mixed” small cell/large cell bronchogenic carcinoma compared to “pure” small cell subtypes. Cancer. 1982;50(12):2894–2902. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

