Bayesian semiparametric mixture Tobit models with left censoring, skewness, and covariate measurement errors
- PMID: 23553914
- PMCID: PMC3773307
- DOI: 10.1002/sim.5799
Bayesian semiparametric mixture Tobit models with left censoring, skewness, and covariate measurement errors
Abstract
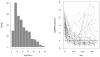
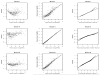
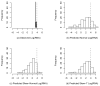
Common problems to many longitudinal HIV/AIDS, cancer, vaccine, and environmental exposure studies are the presence of a lower limit of quantification of an outcome with skewness and time-varying covariates with measurement errors. There has been relatively little work published simultaneously dealing with these features of longitudinal data. In particular, left-censored data falling below a limit of detection may sometimes have a proportion larger than expected under a usually assumed log-normal distribution. In such cases, alternative models, which can account for a high proportion of censored data, should be considered. In this article, we present an extension of the Tobit model that incorporates a mixture of true undetectable observations and those values from a skew-normal distribution for an outcome with possible left censoring and skewness, and covariates with substantial measurement error. To quantify the covariate process, we offer a flexible nonparametric mixed-effects model within the Tobit framework. A Bayesian modeling approach is used to assess the simultaneous impact of left censoring, skewness, and measurement error in covariates on inference. The proposed methods are illustrated using real data from an AIDS clinical study. .
Keywords: measurement error; mixed-effects models; mixture Tobit models; skew distributions.
Copyright © 2013 John Wiley & Sons, Ltd.
Figures


Similar articles
-
New approaches for censored longitudinal data in joint modelling of longitudinal and survival data, with application to HIV vaccine studies.Lifetime Data Anal. 2019 Apr;25(2):229-258. doi: 10.1007/s10985-018-9434-7. Epub 2018 Jun 8. Lifetime Data Anal. 2019. PMID: 29948579 Free PMC article. Review.
-
Bayesian Two-Part Tobit Models with Left-Censoring, Skewness, and Nonignorable Missingness.J Biopharm Stat. 2015;25(4):714-30. doi: 10.1080/10543406.2014.920860. J Biopharm Stat. 2015. PMID: 24905924
-
Bayesian two-part bent-cable Tobit models with skew distributions: Application to AIDS studies.Stat Methods Med Res. 2018 Dec;27(12):3696-3708. doi: 10.1177/0962280217710679. Epub 2017 May 31. Stat Methods Med Res. 2018. PMID: 28560896
-
Mixed-effects joint models with skew-normal distribution for HIV dynamic response with missing and mismeasured time-varying covariate.Int J Biostat. 2012 Nov 26;8(1):/j/ijb.2012.8.issue-1/1557-4679.1426/1557-4679.1426.xml. doi: 10.1515/1557-4679.1426. Int J Biostat. 2012. PMID: 23192055
-
A Bayesian natural cubic B-spline varying coefficient method for non-ignorable dropout.BMC Med Res Methodol. 2020 Oct 7;20(1):250. doi: 10.1186/s12874-020-01135-3. BMC Med Res Methodol. 2020. PMID: 33028226 Free PMC article. Review.
Cited by
-
Flexible longitudinal linear mixed models for multiple censored responses data.Stat Med. 2019 Mar 15;38(6):1074-1102. doi: 10.1002/sim.8017. Epub 2018 Nov 12. Stat Med. 2019. PMID: 30421470 Free PMC article.
-
New approaches for censored longitudinal data in joint modelling of longitudinal and survival data, with application to HIV vaccine studies.Lifetime Data Anal. 2019 Apr;25(2):229-258. doi: 10.1007/s10985-018-9434-7. Epub 2018 Jun 8. Lifetime Data Anal. 2019. PMID: 29948579 Free PMC article. Review.
-
Statistical tests for latent class in censored data due to detection limit.Stat Methods Med Res. 2020 Aug;29(8):2179-2197. doi: 10.1177/0962280219885985. Epub 2019 Nov 18. Stat Methods Med Res. 2020. PMID: 31736411 Free PMC article.
-
Flexible Bayesian semiparametric mixed-effects model for skewed longitudinal data.BMC Med Res Methodol. 2024 Mar 1;24(1):56. doi: 10.1186/s12874-024-02164-y. BMC Med Res Methodol. 2024. PMID: 38429729 Free PMC article.
-
A Bayesian Approach for the Cox Proportional Hazards Model with Covariates Subject to Detection Limit.Int J Stat Med Res. 2014 Jan 31;3(1):32-43. doi: 10.6000/1929-6029.2014.03.01.5. Int J Stat Med Res. 2014. PMID: 24772198 Free PMC article.
References
-
- Paxton WB, Coombs RW, McElrath MJ, Keefer MC, Hughes J, Sinangil F, Chernoff D, Demeter L, Williams B, Corey L. Longitudinal anlysis of quantitative virologic measures in human immunodeficiency virus-infected subjects with ≥ 400 CD4 lymphocytes: Implication for applying measurements to individual patients. Journal of Infectious Disease. 1997;175:247–254. - PubMed
-
- Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, Dragavon J, Peterson G, Hooton TM, Collier AC, Corey L, Koutsky L, Krieger JN. Association between culturable human immunodeficiency virus type-1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: Evidence for compartmentalization of HIV-1 between semen and blood. Journal of Infectious Disease. 1998;177:320–330. - PubMed
-
- Schockmel GA, Yerly S, Perrin L. Detection of Low HIV-1 RNA Levels in Plasma. Journal of Acquired Immune Deficiency Syndromes . 1997;14:179–183. - PubMed
-
- Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, Markowitz M, Ho DD. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. New England Journal of Medicine. 1999;340:1605–1613. - PubMed
-
- Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J, Jr, Gallant JE, Quinn TC, Jackson B, Flexner C, Carson K, Ray S, Persaud D, Siliciano RF. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. Journal of American Medical Association. 2005;293:817–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
