Probing lung microstructure with hyperpolarized noble gas diffusion MRI: theoretical models and experimental results
- PMID: 23554008
- PMCID: PMC6366858
- DOI: 10.1002/mrm.24729
Probing lung microstructure with hyperpolarized noble gas diffusion MRI: theoretical models and experimental results
Abstract
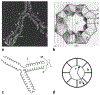
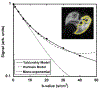
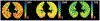
The introduction of hyperpolarized gases ((3)He and (129)Xe) has opened the door to applications for which gaseous agents are uniquely suited-lung MRI. One of the pulmonary applications, diffusion MRI, relies on measuring Brownian motion of inhaled hyperpolarized gas atoms diffusing in lung airspaces. In this article we provide an overview of the theoretical ideas behind hyperpolarized gas diffusion MRI and the results obtained over the decade-long research. We describe a simple technique based on measuring gas apparent diffusion coefficient (ADC) and an advanced technique, in vivo lung morphometry, that quantifies lung microstructure both in terms of Weibel parameters (acinar airways radii and alveolar depth) and standard metrics (mean linear intercept, surface-to-volume ratio, and alveolar density) that are widely used by lung researchers but were previously available only from invasive lung biopsy. This technique has the ability to provide unique three-dimensional tomographic information on lung microstructure from a less than 15 s MRI scan with results that are in good agreement with direct histological measurements. These safe and sensitive diffusion measurements improve our understanding of lung structure and functioning in health and disease, providing a platform for monitoring the efficacy of therapeutic interventions in clinical trials.
Copyright © 2013 Wiley Periodicals, Inc.
Figures





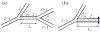

Similar articles
-
Diffusion lung imaging with hyperpolarized gas MRI.NMR Biomed. 2017 Mar;30(3):10.1002/nbm.3448. doi: 10.1002/nbm.3448. Epub 2015 Dec 16. NMR Biomed. 2017. PMID: 26676342 Free PMC article. Review.
-
Lung morphometry with hyperpolarized 129Xe: theoretical background.Magn Reson Med. 2012 Mar;67(3):856-66. doi: 10.1002/mrm.23056. Epub 2011 Jun 28. Magn Reson Med. 2012. PMID: 21713985 Free PMC article.
-
Validating in vivo hyperpolarized 129 Xe diffusion MRI and diffusion morphometry in the mouse lung.Magn Reson Med. 2021 Apr;85(4):2160-2173. doi: 10.1002/mrm.28539. Epub 2020 Oct 5. Magn Reson Med. 2021. PMID: 33017076 Free PMC article.
-
Emphysema Index Based on Hyperpolarized 3He or 129Xe Diffusion MRI: Performance and Comparison with Quantitative CT and Pulmonary Function Tests.Radiology. 2020 Oct;297(1):201-210. doi: 10.1148/radiol.2020192804. Epub 2020 Aug 11. Radiology. 2020. PMID: 32779976 Free PMC article.
-
Translational applications of hyperpolarized 3He and 129Xe.NMR Biomed. 2014 Dec;27(12):1429-38. doi: 10.1002/nbm.3151. Epub 2014 Jun 23. NMR Biomed. 2014. PMID: 24953709 Review.
Cited by
-
Commentary on "The influence of lung airways branching structure and diffusion time on measurements and models of short-range 3He gas MR diffusion".J Magn Reson. 2014 Feb;239:139-42. doi: 10.1016/j.jmr.2013.09.019. Epub 2013 Nov 16. J Magn Reson. 2014. PMID: 24314822 Free PMC article.
-
Alveolar Airspace Size in Healthy and Diseased Infant Lungs Measured via Hyperpolarized 3He Gas Diffusion Magnetic Resonance Imaging.Neonatology. 2020;117(6):704-712. doi: 10.1159/000511084. Epub 2020 Nov 11. Neonatology. 2020. PMID: 33176330 Free PMC article.
-
Hyperpolarized gas MRI in pulmonology.Br J Radiol. 2018 Apr;91(1084):20170647. doi: 10.1259/bjr.20170647. Epub 2018 Jan 22. Br J Radiol. 2018. PMID: 29271239 Free PMC article. Review.
-
Acquiring Hyperpolarized 129Xe Magnetic Resonance Images of Lung Ventilation.J Vis Exp. 2023 Nov 21;(201):10.3791/65982. doi: 10.3791/65982. J Vis Exp. 2023. PMID: 38078603 Free PMC article.
-
In vivo methods and applications of xenon-129 magnetic resonance.Prog Nucl Magn Reson Spectrosc. 2021 Feb;122:42-62. doi: 10.1016/j.pnmrs.2020.11.002. Epub 2020 Dec 9. Prog Nucl Magn Reson Spectrosc. 2021. PMID: 33632417 Free PMC article. Review.
References
-
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. - PubMed
-
- Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol 1967;22:95–108. - PubMed
-
- Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. New Engl J Med 1968;278:1355–1360. - PubMed
-
- Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol 1983;55:1733–1742. - PubMed
-
- Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol 1992;72:1016–1023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

