Amyloid precursor protein controls cholesterol turnover needed for neuronal activity
- PMID: 23554170
- PMCID: PMC3628100
- DOI: 10.1002/emmm.201202215
Amyloid precursor protein controls cholesterol turnover needed for neuronal activity
Abstract
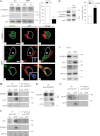
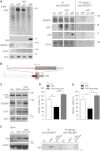
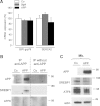
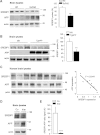
Perturbation of lipid metabolism favours progression of Alzheimer disease, in which processing of Amyloid Precursor Protein (APP) has important implications. APP cleavage is tightly regulated by cholesterol and APP fragments regulate lipid homeostasis. Here, we investigated whether up or down regulation of full-length APP expression affected neuronal lipid metabolism. Expression of APP decreased HMG-CoA reductase (HMGCR)-mediated cholesterol biosynthesis and SREBP mRNA levels, while its down regulation had opposite effects. APP and SREBP1 co-immunoprecipitated and co-localized in the Golgi. This interaction prevented Site-2 protease-mediated processing of SREBP1, leading to inhibition of transcription of its target genes. A GXXXG motif in APP sequence was critical for regulation of HMGCR expression. In astrocytes, APP and SREBP1 did not interact nor did APP affect cholesterol biosynthesis. Neuronal expression of APP decreased both HMGCR and cholesterol 24-hydroxylase mRNA levels and consequently cholesterol turnover, leading to inhibition of neuronal activity, which was rescued by geranylgeraniol, generated in the mevalonate pathway, in both APP expressing and mevastatin treated neurons. We conclude that APP controls cholesterol turnover needed for neuronal activity.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures



References
-
- Aydin D, Weyer SW, Muller UC. Functions of the APP gene family in the nervous system: insights from mouse models. Exp Brain Res. 2012;217:423–434. - PubMed
-
- Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol. 2007;19:215–222. - PubMed
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

