Induction of circularly polarized electroluminescence from an achiral light-emitting polymer via a chiral small-molecule dopant
- PMID: 23554220
- PMCID: PMC3659407
- DOI: 10.1002/adma.201204961
Induction of circularly polarized electroluminescence from an achiral light-emitting polymer via a chiral small-molecule dopant
Abstract
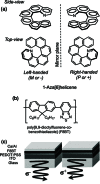
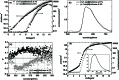
By simply doping the conventional light-emitting polymer F8BT with a helically chiral aromatic molecule, it is shown that substantial levels of CP-electroluminescence can be generated directly. Both photoluminescent and electroluminescent emission from the polymer are observed to become circularly polarized, with the sign of the CP emission directly determined by the handedness of the dopant.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Schadt M. Annu. Rev. Mater. Sci. 1997;27:305.
-
- Sherson JF, Krauter H, Olsson RK, Julsgaard B, Hammerer K, Cirac I, Polzik ES. Nature. 2006;443:557. - PubMed
-
- Wagenknecht C, Li C-M, Reingruber A, Bao X-H, Goebel A, Chen Y–A, Zhang Q, Chen K, Pan J-W. Nat. Photonics. 2010;4:549.
-
- Farshchi R, Ramsteiner M, Herfort J, Tahraoui A, Grahn HT. Appl. Phys. Lett. 2011;98:162508.
-
- Grell M, Oda M, Whitehead KS, Asimakis A, Neher D, Bradley DDC. Adv. Mater. 2001;13:577.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

