Perivascular cells in blood vessel regeneration
- PMID: 23554249
- PMCID: PMC3743428
- DOI: 10.1002/biot.201200199
Perivascular cells in blood vessel regeneration
Abstract
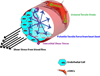
Vascular engineering seeks to design and construct functional blood vessels comprising endothelial cells (ECs) and perivascular cells (PCs), with the ultimate goal of clinical translation. While EC behavior has been extensively investigated, PCs play an equally significant role in the development of novel regenerative strategies, providing functionality and stability to vessels. The two major classes of PCs are vascular smooth muscle cells (vSMCs) and pericytes; vSMCs can be further sub-classified as either contractile or synthetic. The inclusion of these cell types is crucial for successful regeneration of blood vessels. Furthermore, understanding distinctions between vSMCs and pericytes will enable improved therapeutics in a tissue-specific manner. Here we focus on the approaches and challenges facing the use of PCs in vascular regeneration, including their characteristics, stem cell sources, and interactions with ECs. Finally, we discuss biochemical and microRNA (miR) regulators of PC behavior and engineering approaches that mimic various cues affecting PC function.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. - PubMed
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2002.
-
- Armulik A, Genove G, Mae M, Nisancioglu MH, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

