Uterine vasculature remodeling in human pregnancy involves functional macrochimerism by endothelial colony forming cells of fetal origin
- PMID: 23554274
- PMCID: PMC3813980
- DOI: 10.1002/stem.1385
Uterine vasculature remodeling in human pregnancy involves functional macrochimerism by endothelial colony forming cells of fetal origin
Abstract
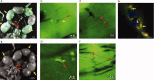
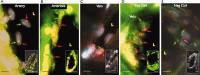
The potency of adult-derived circulating progenitor endothelial colony forming cells (ECFCs) is drastically surpassed by their fetal counterparts. Human pregnancy is associated with robust intensification of blood flow and vascular expansion in the uterus, crucial for placental perfusion and fetal supply. Here, we investigate whether fetal ECFCs transmigrate to maternal bloodstream and home to locations of maternal vasculogenesis, primarily the pregnant uterus. In the first instance, endothelial-like cells, originating from mouse fetuses expressing paternal eGFP, were identified within uterine endothelia. Subsequently, LacZ or enhanced green fluorescent protein (eGFP)-labeled human fetal ECFCs, transplanted into immunodeficient (NOD/SCID) fetuses on D15.5 pregnancy, showed similar integration into the mouse uterus by term. Mature endothelial controls (human umbilical vein endothelial cells), similarly introduced, were unequivocally absent. In humans, SRY was detected in 6 of 12 myometrial microvessels obtained from women delivering male babies. The copy number was calculated at 175 [IQR 149-471] fetal cells per millimeter square endothelium, constituting 12.5% of maternal vessel lumina. Cross-sections of similar human vessels, hybridized for Y-chromosome, positively identified endothelial-associated fetal cells. It appears that through ECFC donation, fetuses assist maternal uterine vascular expansion in pregnancy, potentiating placental perfusion and consequently their own fetal supply. In addition to fetal growth, this cellular mechanism holds implications for materno-fetal immune interactions and long-term maternal vascular health.
Keywords: Chimerism; Circulation; Maternal-Fetal Exchange; Neovascularization; Physiologic; Uteroplacenta.
Copyright © 2013 AlphaMed Press.
Figures


References
-
- Berg CJ, Mackay AP, Qin C, et al. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol. 2009;113:1075–1081. - PubMed
-
- Mikolajczyk RT, Zhang J, Betran AP, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377:1855–1861. - PubMed
-
- Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(suppl 3):332–336. - PubMed
-
- Barker DJ, Osmond C, Forsen TJ, et al. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

