Connexin43 is dispensable for phagocytosis
- PMID: 23554311
- PMCID: PMC3633682
- DOI: 10.4049/jimmunol.1202884
Connexin43 is dispensable for phagocytosis
Abstract
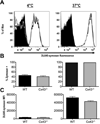
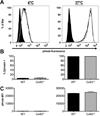
Macrophages that lack connexin43 (Cx43), a gap junction protein, have been reported to exhibit dramatic deficiencies in phagocytosis. In this study, we revisit these findings using well-characterized macrophage populations. Cx43 knockout (Cx43(-/-)) mice die soon after birth, making the harvest of macrophages from adult Cx43(-/-) mice problematic. To overcome this obstacle, we used several strategies: mice heterozygous for the deletion of Cx43 were crossed to produce Cx43(+/+) (wild type [WT]) and Cx43(-/-) fetuses. Cells isolated from 12- to 14-d fetal livers were used to reconstitute irradiated recipient animals. After reconstitution, thioglycollate-elicited macrophages were collected by peritoneal lavage and bone marrow was harvested. Bone marrow cells and, alternatively, fetal liver cells were cultured in media containing M-CSF for 7-10 d, resulting in populations of cells that were >95% macrophages based on flow cytometry. Phagocytic uptake was detected using flow cytometric and microscopic techniques. Quantification of phagocytic uptake of IgG-opsonized sheep erythrocytes, zymosan particles, and Listeria monocytogenes failed to show any significant difference between WT and Cx43(-/-) macrophages. Furthermore, the use of particles labeled with pH-sensitive dyes showed equivalent acidification of phagosomes in both WT and Cx43(-/-) macrophages. Our findings suggest that modulation of Cx43 levels in cultured macrophages does not have a significant impact on phagocytosis.
Figures




References
-
- Pang B, Neijssen J, Qiao X, Janssen L, Janssen H, Lippuner C, Neefjes J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J Immunol. 2009;183:1083–1090. - PubMed
-
- Neijssen J, Pang B, Neefjes J. Gap junction-mediated intercellular communication in the immune system. Prog Biophys Mol Biol. 2007;94:207–218. - PubMed
-
- Nguyen TD, Taffet SM. A model system to study Connexin 43 in the immune system. Mol Immunol. 2009;46:2938–2946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

