Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents
- PMID: 23554417
- PMCID: PMC3623378
- DOI: 10.1128/CMR.00092-12
Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents
Abstract
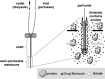
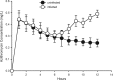
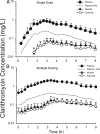
For the optimization of dosing regimens of anti-infective agents, it is imperative to have a good understanding of pharmacokinetics (PK) and pharmacodynamics (PD). Whenever possible, drug efficacy needs to be related to unbound concentrations at the site of action. For anti-infective drugs, the infection site is typically located outside plasma, and a drug must diffuse through capillary membranes to reach its target. Disease- and drug-related factors can contribute to differential tissue distribution. As a result, the assumption that the plasma concentration of drugs represents a suitable surrogate of tissue concentrations may lead to erroneous conclusions. Quantifying drug exposure in tissues represents an opportunity to relate the pharmacologically active concentrations to an observed pharmacodynamic parameter, such as the MIC. Selection of an appropriate specimen to sample and the advantages and limitations of the available sampling techniques require careful consideration. Ultimately, the goal will be to assess the appropriateness of a drug and dosing regimen for a specific pathogen and infection.
Figures




References
-
- Kratochwil NA, Huber W, Müller F, Kansy M, Gerber PR. 2002. Predicting plasma protein binding of drugs: a new approach. Biochem. Pharmacol. 64:1355–1374 - PubMed
-
- Craig W, Welling P. 1977. Protein binding of antimicrobials: clinical pharmacokinetic and therapeutic implications. Clin. Pharmacokinet. 2:252–268 - PubMed
-
- Toutain P, Bousquet-Melou A. 2002. Free drug fraction vs free drug concentration: a matter of frequent confusion. J. Vet. Pharmacol. Ther. 25:460–463 - PubMed
-
- Perry T, Schentag JJ. 2001. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin. Pharmacokinet. 40:685–694 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

