Potassium conductance dynamics confer robust spike-time precision in a neuromorphic model of the auditory brain stem
- PMID: 23554436
- PMCID: PMC3727074
- DOI: 10.1152/jn.00433.2012
Potassium conductance dynamics confer robust spike-time precision in a neuromorphic model of the auditory brain stem
Abstract
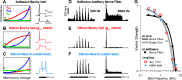
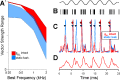
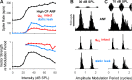
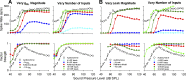
A fundamental question in neuroscience is how neurons perform precise operations despite inherent variability. This question also applies to neuromorphic engineering, where low-power microchips emulate the brain using large populations of diverse silicon neurons. Biological neurons in the auditory pathway display precise spike timing, critical for sound localization and interpretation of complex waveforms such as speech, even though they are a heterogeneous population. Silicon neurons are also heterogeneous, due to a key design constraint in neuromorphic engineering: smaller transistors offer lower power consumption and more neurons per unit area of silicon, but also more variability between transistors and thus between silicon neurons. Utilizing this variability in a neuromorphic model of the auditory brain stem with 1,080 silicon neurons, we found that a low-voltage-activated potassium conductance (g(KL)) enables precise spike timing via two mechanisms: statically reducing the resting membrane time constant and dynamically suppressing late synaptic inputs. The relative contribution of these two mechanisms is unknown because blocking g(KL) in vitro eliminates dynamic adaptation but also lengthens the membrane time constant. We replaced g(KL) with a static leak in silico to recover the short membrane time constant and found that silicon neurons could mimic the spike-time precision of their biological counterparts, but only over a narrow range of stimulus intensities and biophysical parameters. The dynamics of g(KL) were required for precise spike timing robust to stimulus variation across a heterogeneous population of silicon neurons, thus explaining how neural and neuromorphic systems may perform precise operations despite inherent variability.
Keywords: bushy cells; gKL; heterogeneity; phase locking; silicon neuron.
Figures



References
-
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol 447: 331–350, 2002 - PubMed
-
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 86: 2299–2311, 2001 - PubMed
-
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli”. J Acoust Soc Am 112: 1026–1036, 2002 - PubMed
-
- Blackburn CC, Sachs MB. Classification of unit types in the anteroventral cochlear nucleus: PST histograms and regularity analysis. J Neurophysiol 62: 1303–1329, 1989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

