Disparate host immunity to Mycobacterium avium subsp. paratuberculosis antigens in calves inoculated with M. avium subsp. paratuberculosis, M. avium subsp. avium, M. kansasii, and M. bovis
- PMID: 23554467
- PMCID: PMC3675962
- DOI: 10.1128/CVI.00051-13
Disparate host immunity to Mycobacterium avium subsp. paratuberculosis antigens in calves inoculated with M. avium subsp. paratuberculosis, M. avium subsp. avium, M. kansasii, and M. bovis
Abstract
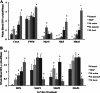
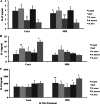
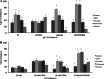
The cross-reactivity of mycobacterial antigens in immune-based diagnostic assays has been a major concern and a criticism of the current tests that are used for the detection of paratuberculosis. In the present study, Mycobacterium avium subsp. paratuberculosis recombinant proteins were evaluated for antigenic specificity compared to a whole-cell sonicate preparation (MPS). Measures of cell-mediated immunity to M. avium subsp. paratuberculosis antigens were compared in calves inoculated with live M. avium subsp. paratuberculosis, M. avium subsp. avium (M. avium), Mycobacterium kansasii, or Mycobacterium bovis. Gamma interferon (IFN-γ) responses to MPS were observed in all calves that were exposed to mycobacteria compared to control calves at 4 months postinfection. Pooled recombinant M. avium subsp. paratuberculosis proteins also elicited nonspecific IFN-γ responses in inoculated calves, with the exception of calves infected with M. bovis. M. avium subsp. paratuberculosis proteins failed to elicit antigen-specific responses for the majority of immune measures; however, the expression of CD25 and CD26 was upregulated on CD4, CD8, gamma/delta (γδ) T, and B cells for the calves that were inoculated with either M. avium subsp. paratuberculosis or M. avium after antigen stimulation of the cells. Stimulation with MPS also resulted in the increased expression of CD26 on CD45RO(+) CD25(+) T cells from calves inoculated with M. avium subsp. paratuberculosis and M. avium. Although recombinant proteins failed to elicit specific responses for the calves inoculated with M. avium subsp. paratuberculosis, the differences in immune responses to M. avium subsp. paratuberculosis antigens were dependent upon mycobacterial exposure. The results demonstrated a close alignment in immune responses between calves inoculated with M. avium subsp. paratuberculosis and those inoculated with M. avium that were somewhat disparate from the responses in calves infected with M. bovis, suggesting that the biology of mycobacterial infection plays an important role in diagnosis.
Figures


Similar articles
-
Comparative cellular immune responses in calves after infection with Mycobacterium avium subsp. paratuberculosis, M. avium subsp. avium, M. kansasii and M. bovis.Vet Immunol Immunopathol. 2021 Jul;237:110268. doi: 10.1016/j.vetimm.2021.110268. Epub 2021 May 19. Vet Immunol Immunopathol. 2021. PMID: 34023615
-
Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis.Clin Diagn Lab Immunol. 2004 Jul;11(4):729-35. doi: 10.1128/CDLI.11.4.729-735.2004. Clin Diagn Lab Immunol. 2004. PMID: 15242948 Free PMC article.
-
Early immune markers associated with Mycobacterium avium subsp. paratuberculosis infection in a neonatal calf model.Clin Vaccine Immunol. 2011 Mar;18(3):393-405. doi: 10.1128/CVI.00359-10. Epub 2011 Jan 12. Clin Vaccine Immunol. 2011. PMID: 21228140 Free PMC article.
-
Advances in Understanding of the Immune Response to Mycobacterial Pathogens and Vaccines through Use of Cattle and Mycobacterium avium subsp. paratuberculosis as a Prototypic Mycobacterial Pathogen.Vaccines (Basel). 2021 Sep 26;9(10):1085. doi: 10.3390/vaccines9101085. Vaccines (Basel). 2021. PMID: 34696193 Free PMC article. Review.
-
No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis.Infect Immun. 2013 Nov;81(11):3960-5. doi: 10.1128/IAI.00575-13. Epub 2013 Aug 12. Infect Immun. 2013. PMID: 23940208 Free PMC article. Review.
Cited by
-
Recombinant BCG-Prime and DNA-Boost Immunization Confers Mice with Enhanced Protection against Mycobacterium kansasii.Vaccines (Basel). 2021 Nov 1;9(11):1260. doi: 10.3390/vaccines9111260. Vaccines (Basel). 2021. PMID: 34835191 Free PMC article.
-
Identification of Novel Antigens Recognized by Serum Antibodies in Bovine Tuberculosis.Clin Vaccine Immunol. 2017 Dec 5;24(12):e00259-17. doi: 10.1128/CVI.00259-17. Print 2017 Dec. Clin Vaccine Immunol. 2017. PMID: 28978510 Free PMC article.
-
Transcriptome Profiling of Bovine Macrophages Infected by Mycobacterium avium spp. paratuberculosis Depicts Foam Cell and Innate Immune Tolerance Phenotypes.Front Immunol. 2020 Jan 8;10:2874. doi: 10.3389/fimmu.2019.02874. eCollection 2019. Front Immunol. 2020. PMID: 31969876 Free PMC article.
-
Interferon-γ Response of Mycobacterium avium subsp. paratuberculosis Infected Goats to Recombinant and Synthetic Mycobacterial Antigens.Front Vet Sci. 2021 Mar 26;8:645251. doi: 10.3389/fvets.2021.645251. eCollection 2021. Front Vet Sci. 2021. PMID: 33842578 Free PMC article.
-
Divergent Antigen-Specific Cellular Immune Responses during Asymptomatic Subclinical and Clinical States of Disease in Cows Naturally Infected with Mycobacterium avium subsp. paratuberculosis.Infect Immun. 2019 Dec 17;88(1):e00650-19. doi: 10.1128/IAI.00650-19. Print 2019 Dec 17. Infect Immun. 2019. PMID: 31611273 Free PMC article.
References
-
- Osterstock JB, Fosgate GT, Norby B, Manning EJ, Collins MT, Roussel AJ. 2007. Contribution of environmental mycobacteria for false-positive serum ELISA results for paratuberculosis. J. Am. Vet. Med. Assoc. 230:896–901 - PubMed
-
- Falkinham JO., III 2002. Nontuberculous mycobacteria in the environment. Clin. Chest Med. 23:529–551 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

