Millisecond stimulus onset-asynchrony enhances information about components in an odor mixture
- PMID: 23554487
- PMCID: PMC6618935
- DOI: 10.1523/JNEUROSCI.5838-12.2013
Millisecond stimulus onset-asynchrony enhances information about components in an odor mixture
Abstract
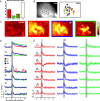
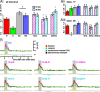
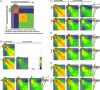
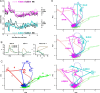
Airborne odorants rarely occur as pure, isolated stimuli. In a natural environment, odorants that intermingle from multiple sources create mixtures in which the onset and offset of odor components are asynchronous. Odor mixtures are known to elicit interactions in both behavioral and physiological responses, changing the perceptive quality of mixtures compared with the components. However, relevant odors need to be segregated from a distractive background. Honeybees (Apis mellifera) can use stimulus onset asynchrony of as little as 6 ms to segregate learned odor components within a mixture. Using in vivo calcium imaging of projection neurons in the honeybee, we studied neuronal mechanisms of odor-background segregation based on stimulus onset asynchrony in the antennal lobe. We found that asynchronous mixtures elicit response patterns that are different from their synchronous counterpart: the responses to asynchronous mixtures contain more information about the constituent components. With longer onset shifts, more features of the components were present in the mixture response patterns. Moreover, we found that the processing of asynchronous mixtures activated more inhibitory interactions than the processing of synchronous mixtures. This study provides evidence of neuronal mechanisms that underlie odor-object segregation on a timescale much faster than found for mammals.
Figures


References
-
- Baker TC, Fadamiro HY, Cosse AA. Moth uses fine tuning for odour resolution [letter] Nature. 1998;393:530.
-
- Bronkhorst AW. The cocktail party phenomenon: a review of research on speech intelligibility in multiple-talker conditions. Acta Acustica united with Acustica. 2000;86:117–128.
-
- Broome BM, Jayaraman V, Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51:467–482. - PubMed
-
- Chandra S., Smith B. H. An analysis of synthetic processing of odor mixtures in the honeybee (Apis mellifera) J Exp Biol. 1998;201(Pt 22):3113–3121. - PubMed
-
- Coureaud G, Hamdani Y, Schaal B, Thomas-Danguin T. Elemental and configural processing of odour mixtures in the newborn rabbit. J Exp Biol. 2009;212:2525–2531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
