Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities
- PMID: 23554489
- PMCID: PMC3656411
- DOI: 10.1523/JNEUROSCI.0035-13.2013
Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities
Abstract
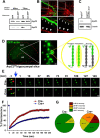
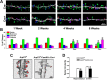
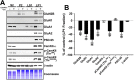
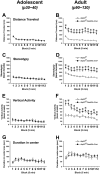
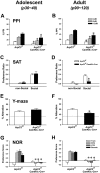
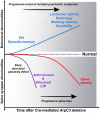
Despite evidence for a strong genetic contribution to several major psychiatric disorders, individual candidate genes account for only a small fraction of these disorders, leading to the suggestion that multigenetic pathways may be involved. Several known genetic risk factors for psychiatric disease are related to the regulation of actin polymerization, which plays a key role in synaptic plasticity. To gain insight into and test the possible pathogenetic role of this pathway, we designed a conditional knock-out of the Arp2/3 complex, a conserved final output for actin signaling pathways that orchestrates de novo actin polymerization. Here we report that postnatal loss of the Arp2/3 subunit ArpC3 in forebrain excitatory neurons leads to an asymmetric structural plasticity of dendritic spines, followed by a progressive loss of spine synapses. This progression of synaptic deficits corresponds with an evolution of distinct cognitive, psychomotor, and social disturbances as the mice age. Together, these results point to the dysfunction of actin signaling, specifically that which converges to regulate Arp2/3, as an important cellular pathway that may contribute to the etiology of complex psychiatric disorders.
Figures


References
-
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. - PubMed
-
- Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. - PubMed
-
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Müller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. - PMC - PubMed
-
- Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol. 2011;95:275–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
