Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons
- PMID: 23554490
- PMCID: PMC3668454
- DOI: 10.1523/JNEUROSCI.0216-13.2013
Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons
Abstract
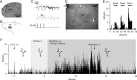
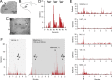
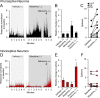
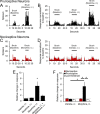
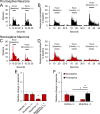
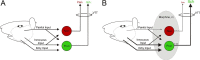
Intrathecal application of morphine is among the most powerful methods used to treat severe chronic pain. However, this approach commonly produces itch sufficiently severe that patients are forced to choose between relief of pain or itch. The neuronal populations responsible for processing and transmitting information underlying itch caused by intrathecal application of morphine have not been identified and characterized. We describe two populations of antidromically identified trigeminothalamic tract (VTT) neurons in anesthetized rats that are differentially affected by morphine and explain several aspects of opioid-induced itch and analgesia. We found that intrathecal application of morphine increased ongoing activity of itch-responsive VTT neurons. In addition, intrathecal application of morphine increased responses to pruritogens injected into the skin and greatly heightened responses to innocuous mechanical stimuli. In contrast, the ongoing activity and responses to noxious pinches in nociceptive VTT neurons were frequently inhibited by the same dose of morphine. These results reveal that i.t. application of morphine affects specific subpopulations of VTT neurons in ways that may produce itch, hyperknesis, alloknesis, and analgesia.
Figures
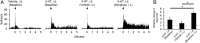
Similar articles
-
Characterization of pruriceptive trigeminothalamic tract neurons in rats.J Neurophysiol. 2014 Apr;111(8):1574-89. doi: 10.1152/jn.00668.2013. Epub 2014 Jan 29. J Neurophysiol. 2014. PMID: 24478156 Free PMC article.
-
Superficial dorsal horn neurons identified by intracutaneous histamine: chemonociceptive responses and modulation by morphine.J Neurophysiol. 2000 Aug;84(2):616-27. doi: 10.1152/jn.2000.84.2.616. J Neurophysiol. 2000. PMID: 10938290
-
Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition.Brain. 2021 Mar 3;144(2):665-681. doi: 10.1093/brain/awaa430. Brain. 2021. PMID: 33367648
-
Drug-related side effects of long-term intrathecal morphine therapy.Pain Physician. 2007 Mar;10(2):357-66. Pain Physician. 2007. PMID: 17387357 Review.
-
The Effect of Intrathecal Morphine Dose on Outcomes After Elective Cesarean Delivery: A Meta-Analysis.Anesth Analg. 2016 Jul;123(1):154-64. doi: 10.1213/ANE.0000000000001255. Anesth Analg. 2016. PMID: 27089000 Review.
Cited by
-
Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates.Br J Pharmacol. 2015 Jul;172(13):3302-12. doi: 10.1111/bph.13124. Epub 2015 Apr 24. Br J Pharmacol. 2015. PMID: 25752320 Free PMC article.
-
Neural processing of itch.Neuroscience. 2013 Oct 10;250:697-714. doi: 10.1016/j.neuroscience.2013.07.035. Epub 2013 Jul 24. Neuroscience. 2013. PMID: 23891755 Free PMC article. Review.
-
Intracerebroventricular Coadministration of Protoxin-II and Trace Elements in Rats Enhances the Analgesic Effect of the 1.7 Voltage-Gate Sodium Channel Blocker.Biomed Res Int. 2019 Dec 28;2019:8057803. doi: 10.1155/2019/8057803. eCollection 2019. Biomed Res Int. 2019. PMID: 32090064 Free PMC article.
-
Insensitivity to pain induced by a potent selective closed-state Nav1.7 inhibitor.Sci Rep. 2017 Jan 3;7:39662. doi: 10.1038/srep39662. Sci Rep. 2017. PMID: 28045073 Free PMC article.
-
Spinal cord projection neurons: a superficial, and also deep, analysis.Curr Opin Physiol. 2019 Oct;11:109-115. doi: 10.1016/j.cophys.2019.10.002. Epub 2019 Oct 10. Curr Opin Physiol. 2019. PMID: 32864531 Free PMC article.
References
-
- Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. - PubMed
-
- Baraka A, Noueihid R, Hajj S. Intrathecal injection of morphine for obstetric analgesia. Anesthesiology. 1981;54:136–140. - PubMed
-
- Baraka A, Maktabi M, Noueihid R. Epidural meperidine-bupivacaine for obstetric analgesia. Anesth Analg. 1982;61:652–656. - PubMed
-
- Berendsen HH, Broekkamp CL. A peripheral 5-HT1D-like receptor involved in serotonergic induced hindlimb scratching in rats. Eur J Pharmacol. 1991;194:201–208. - PubMed
-
- Bromage PR, Camporesi EM, Durant PA, Nielsen CH. Nonrespiratory side effects of epidural morphine. Anesth Analg. 1982;61:490–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
