A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans
- PMID: 23554491
- PMCID: PMC6618909
- DOI: 10.1523/JNEUROSCI.4023-12.2013
A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans
Abstract
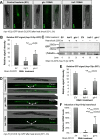
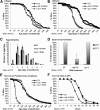
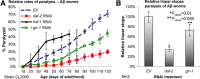
In the nematode Caenorhabditis elegans, the heat shock response (HSR) is regulated at the organismal level by a network of thermosensory neurons that senses elevated temperatures and activates the HSR in remote tissues. Which neuronal receptors are required for this signaling mechanism and in which neurons they function are largely unanswered questions. Here we used worms that were engineered to exhibit RNA interference hypersensitivity in neurons to screen for neuronal receptors that are required for the activation of the HSR and identified a putative G-protein coupled receptor (GPCR) as a novel key component of this mechanism. This gene, which we termed GPCR thermal receptor 1 (gtr-1), is expressed in chemosensory neurons and has no role in heat sensing but is critically required for the induction of genes that encode heat shock proteins in non-neural tissues upon exposure to heat. Surprisingly, the knock-down of gtr-1 by RNA interference protected worms expressing the Alzheimer's-disease-linked aggregative peptide Aβ3-42 from proteotoxicity but had no effect on lifespan. This study provides several novel insights: (1) it shows that chemosensory neurons play important roles in the nematode's HSR-regulating mechanism, (2) it shows that lifespan and heat stress resistance are separable, and (3) it strengthens the emerging notion that the ability to respond to heat comes at the expense of protein homeostasis (proteostasis).
Figures

Similar articles
-
Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans.Nat Neurosci. 2011 Jun 26;14(8):984-92. doi: 10.1038/nn.2854. Nat Neurosci. 2011. PMID: 21706021
-
Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons.Science. 2008 May 9;320(5877):811-4. doi: 10.1126/science.1156093. Science. 2008. PMID: 18467592 Free PMC article.
-
Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1.Aging Cell. 2014 Jun;13(3):419-30. doi: 10.1111/acel.12189. Epub 2014 Jan 9. Aging Cell. 2014. PMID: 24314125 Free PMC article.
-
Transcellular chaperone signaling: an organismal strategy for integrated cell stress responses.J Exp Biol. 2014 Jan 1;217(Pt 1):129-36. doi: 10.1242/jeb.091249. J Exp Biol. 2014. PMID: 24353212 Free PMC article. Review.
-
The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans.Int J Mol Sci. 2022 Nov 28;23(23):14907. doi: 10.3390/ijms232314907. Int J Mol Sci. 2022. PMID: 36499234 Free PMC article. Review.
Cited by
-
A short peptide protects from age-onset proteotoxicity.Aging Cell. 2023 Dec;22(12):e14013. doi: 10.1111/acel.14013. Epub 2023 Oct 27. Aging Cell. 2023. PMID: 37897137 Free PMC article.
-
Experience Modulates the Reproductive Response to Heat Stress in C. elegans via Multiple Physiological Processes.PLoS One. 2015 Dec 29;10(12):e0145925. doi: 10.1371/journal.pone.0145925. eCollection 2015. PLoS One. 2015. PMID: 26713620 Free PMC article.
-
A Differentiation Transcription Factor Establishes Muscle-Specific Proteostasis in Caenorhabditis elegans.PLoS Genet. 2016 Dec 30;12(12):e1006531. doi: 10.1371/journal.pgen.1006531. eCollection 2016 Dec. PLoS Genet. 2016. PMID: 28036392 Free PMC article.
-
Modulation of caveolae by insulin/IGF-1 signaling regulates aging of Caenorhabditis elegans.EMBO Rep. 2018 Aug;19(8):e45673. doi: 10.15252/embr.201745673. Epub 2018 Jun 26. EMBO Rep. 2018. PMID: 29945933 Free PMC article.
-
Gonadotropin-releasing hormone-like receptor 2 inversely regulates somatic proteostasis and reproduction in Caenorhabditis elegans.Front Cell Dev Biol. 2022 Aug 29;10:951199. doi: 10.3389/fcell.2022.951199. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36105349 Free PMC article.
References
-
- Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. - PubMed
-
- Cassata G, Kagoshima H, Andachi Y, Kohara Y, Dürrenberger MB, Hall DH, Bürglin TR. The LIM homeobox gene ceh-14 confers thermosensory function to the AFD neurons in Caenorhabditis elegans. Neuron. 2000;25:587–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
