Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus
- PMID: 23554498
- PMCID: PMC3736310
- DOI: 10.1523/JNEUROSCI.2437-12.2013
Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus
Abstract
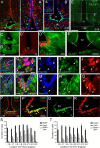
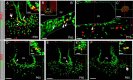
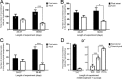
Increasing evidence suggests that neurogenesis occurs in the postnatal and adult mammalian hypothalamus. However, the identity and location of the putative progenitor cells is under much debate, and little is known about the dynamics of neurogenesis in unchallenged brain. Previously, we postulated that Fibroblast growth factor 10-expressing (Fgf10(+)) tanycytes constitute a population of progenitor cells in the mouse hypothalamus. Here, we show that Fgf10(+) tanycytes express markers of neural stem/progenitor cells, divide late into postnatal life, and can generate both neurons and astrocytes in vivo. Stage-specific lineage-tracing of Fgf10(+) tanycytes using Fgf10-creERT2 mice, reveals robust neurogenesis at postnatal day 28 (P28), lasting as late as P60. Furthermore, we present evidence for amplification of Fgf10-lineage traced neural cells within the hypothalamic parenchyma itself. The neuronal descendants of Fgf10(+) tanycytes predominantly populate the arcuate nucleus, a subset of which express the orexigenic neuronal marker, Neuropeptide-Y, and respond to fasting and leptin-induced signaling. These studies provide direct evidence in support of hypothalamic neurogenesis during late postnatal and adult life, and identify Fgf10(+) tanycytes as a source of parenchymal neurons with putative roles in appetite and energy balance.
Figures





Similar articles
-
Fibroblast growth factor 10 is a negative regulator of postnatal neurogenesis in the mouse hypothalamus.Development. 2020 Jul 13;147(13):dev180950. doi: 10.1242/dev.180950. Development. 2020. PMID: 32661019 Free PMC article.
-
Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche.Nat Neurosci. 2012 Mar 25;15(5):700-2. doi: 10.1038/nn.3079. Nat Neurosci. 2012. PMID: 22446882 Free PMC article.
-
Transient expression of neuropeptide W in postnatal mouse hypothalamus--a putative regulator of energy homeostasis.Neuroscience. 2015 Aug 20;301:323-37. doi: 10.1016/j.neuroscience.2015.06.014. Epub 2015 Jun 11. Neuroscience. 2015. PMID: 26073698
-
Irx3 and Irx5 - Novel Regulatory Factors of Postnatal Hypothalamic Neurogenesis.Front Neurosci. 2021 Nov 2;15:763856. doi: 10.3389/fnins.2021.763856. eCollection 2021. Front Neurosci. 2021. PMID: 34795556 Free PMC article. Review.
-
Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions.Front Neurosci. 2015 Oct 29;9:387. doi: 10.3389/fnins.2015.00387. eCollection 2015. Front Neurosci. 2015. PMID: 26578855 Free PMC article. Review.
Cited by
-
Role of non-neuronal cells in body weight and appetite control.Front Endocrinol (Lausanne). 2015 Mar 26;6:42. doi: 10.3389/fendo.2015.00042. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25859240 Free PMC article. Review.
-
Buttressing a balanced brain: Target-derived FGF signaling regulates excitatory/inhibitory tone and adult neurogenesis within the maturating hippocampal network.Neurogenesis (Austin). 2016 Apr 12;3(1):e1168504. doi: 10.1080/23262133.2016.1168504. eCollection 2016. Neurogenesis (Austin). 2016. PMID: 27605441 Free PMC article.
-
Characterisation of endogenous players in fibroblast growth factor-regulated functions of hypothalamic tanycytes and energy-balance nuclei.J Neuroendocrinol. 2019 Aug;31(8):e12750. doi: 10.1111/jne.12750. Epub 2019 Jul 8. J Neuroendocrinol. 2019. PMID: 31111569 Free PMC article.
-
Oestradiol and diet modulate energy homeostasis and hypothalamic neurogenesis in the adult female mouse.J Neuroendocrinol. 2014 Nov;26(11):805-16. doi: 10.1111/jne.12206. J Neuroendocrinol. 2014. PMID: 25182179 Free PMC article.
-
Walking along the Fibroblast Growth Factor 10 Route: A Key Pathway to Understand the Control and Regulation of Epithelial and Mesenchymal Cell-Lineage Formation during Lung Development and Repair after Injury.Scientifica (Cairo). 2014;2014:538379. doi: 10.1155/2014/538379. Epub 2014 Sep 11. Scientifica (Cairo). 2014. PMID: 25298902 Free PMC article. Review.
References
-
- Antoine M, Reimers K, Wirz W, Gressner AM, Müller R, Kiefer P. Fibroblast growth factor 3, a protein with a dual subcellular fate, is interacting with human ribosomal protein S2. Biochem Biophys Res Commun. 2005;338:1248–1255. - PubMed
-
- Becskei C, Lutz TA, Riediger T. Reduced fasting-induced activation of hypothalamic arcuate neurons is associated with hyperleptinemia and increased leptin sensitivity in obese mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R632–R641. - PubMed
-
- Berger UV, Hediger MA. Differential distribution of the glutamate transporters GLT-1 and GLAST in tanycytes of the third ventricle. J Comp Neurol. 2001;433:101–114. - PubMed
-
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
