PPARα regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving α7 nicotinic acetylcholine receptors
- PMID: 23554501
- PMCID: PMC6618938
- DOI: 10.1523/JNEUROSCI.4647-12.2013
PPARα regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving α7 nicotinic acetylcholine receptors
Abstract
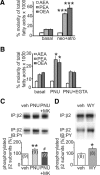
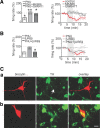
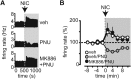
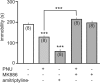
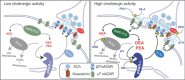
Ventral tegmental area dopamine neurons control reward-driven learning, and their dysregulation can lead to psychiatric disorders. Tonic and phasic activity of these dopaminergic neurons depends on cholinergic tone and activation of nicotinic acetylcholine receptors (nAChRs), particularly those containing the β2 subunit (β2*-nAChRs). Nuclear peroxisome proliferator-activated receptors type-α (PPARα) tonically regulate β2*-nAChRs and thereby control dopamine neuron firing activity. However, it is unknown how and when PPARα endogenous ligands are synthesized by dopamine cells. Using ex vivo and in vivo electrophysiological techniques combined with biochemical and behavioral analysis, we show that activation of α7-nAChRs increases in the rat VTA both the tyrosine phosphorylation of the β2 subunit of nAChRs and the levels of two PPARα endogenous ligands in a Ca(2+)-dependent manner. Accordingly, in vivo production of endogenous PPARα ligands, triggered by α7-nAChR activation, blocks in rats nicotine-induced increased firing activity of dopamine neurons and displays antidepressant-like properties. These data demonstrate that endogenous PPARα ligands are effectors of α7-nAChRs and that their neuromodulatory properties depend on phosphorylation of β2*-nAChRs on VTA dopamine cells. This reveals an autoinhibitory mechanism aimed at reducing dopamine cell overexcitation engaged during hypercholinergic drive. Our results unveil important physiological functions of nAChR/PPARα signaling in dopamine neurons and how behavioral output can change after modifications of this signaling pathway. Overall, the present study suggests PPARα as new therapeutic targets for disorders associated with unbalanced dopamine-acetylcholine systems.
Figures

References
-
- Acker BA, Jacobsen EJ, Rogers BN, Wishka DG, Reitz SC, Piotrowski DW, Myers JK, Wolfe ML, Groppi VE, Thornburgh BA, Tinholt PM, Walters RR, Olson BA, Fitzgerald L, Staton BA, Raub TJ, Krause M, Li KS, Hoffmann WE, Hajos M, et al. Discovery of N+-[(3R,5R)-1-azabicyclo[3.2.1]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide as an agonist of the alpha7 nicotinic acetylcholine receptor: in vitro and in vivo activity. Bioorg Med Chem Lett. 2008;18:3611–3615. - PubMed
-
- Adamczyk P, Golda A, McCreary AC, Filip M, Przegalinski E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. 2008;59:217–228. - PubMed
-
- Albanese A, Minciacchi D. Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. J Comp Neurol. 1983;216:406–420. - PubMed
-
- Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol. 2009;20:286–295. - PubMed
-
- Astarita G, Ahmed F, Piomelli D. Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain. J Lipid Res. 2008;49:48–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
