Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes
- PMID: 23554581
- PMCID: PMC3595214
- DOI: 10.1371/journal.pmed.1001403
Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes
Abstract
Background: Atypical antipsychotic medications are widely prescribed for the adjunctive treatment of depression, yet their total risk-benefit profile is not well understood. We thus conducted a systematic review of the efficacy and safety profiles of atypical antipsychotic medications used for the adjunctive treatment of depression.
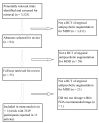
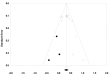
Methods and findings: We included randomized trials comparing adjunctive antipsychotic medication to placebo for treatment-resistant depression in adults. Our literature search (conducted in December 2011 and updated on December 14, 2012) identified 14 short-term trials of aripiprazole, olanzapine/fluoxetine combination (OFC), quetiapine, and risperidone. When possible, we supplemented published literature with data from manufacturers' clinical trial registries and US Food and Drug Administration New Drug Applications. Study duration ranged from 4 to 12 wk. All four drugs had statistically significant effects on remission, as follows: aripiprazole (odds ratio [OR], 2.01; 95% CI, 1.48-2.73), OFC (OR, 1.42; 95% CI, 1.01-2.0), quetiapine (OR, 1.79; 95% CI, 1.33-2.42), and risperidone (OR, 2.37; 95% CI, 1.31-4.30). The number needed to treat (NNT) was 19 for OFC and nine for each other drug. All drugs with the exception of OFC also had statistically significant effects on response rates, as follows: aripiprazole (OR, 2.07; 95% CI, 1.58-2.72; NNT, 7), OFC (OR, 1.30, 95% CI, 0.87-1.93), quetiapine (OR, 1.53, 95% CI, 1.17-2.0; NNT, 10), and risperidone (OR, 1.83, 95% CI, 1.16-2.88; NNT, 8). All four drugs showed statistically significant effects on clinician-rated depression severity measures (Hedges' g ranged from 0.26 to 0.48; mean difference of 2.69 points on the Montgomery-Asberg Depression Rating Scale across drugs). On measures of functioning and quality of life, these medications produced either no benefit or a very small benefit, except for risperidone, which had a small-to-moderate effect on quality of life (g = 0.49). Treatment was linked to several adverse events, including akathisia (aripiprazole), sedation (quetiapine, OFC, and aripiprazole), abnormal metabolic laboratory results (quetiapine and OFC), and weight gain (all four drugs, especially OFC). Shortcomings in study design and data reporting, as well as use of post hoc analyses, may have inflated the apparent benefits of treatment and reduced the apparent incidence of adverse events.
Conclusions: Atypical antipsychotic medications for the adjunctive treatment of depression are efficacious in reducing observer-rated depressive symptoms, but clinicians should interpret these findings cautiously in light of (1) the small-to-moderate-sized benefits, (2) the lack of benefit with regards to quality of life or functional impairment, and (3) the abundant evidence of potential treatment-related harm. Please see later in the article for the Editors' Summary.
Conflict of interest statement
GIS is a member of Healthy Skepticism and holds shares of less than $10,000 in a mutual fund (Vanguard Health Care) that invests heavily in pharmaceutical firms. NZR is a member of both the National Physicians Alliance and Healthy Skepticism. NZR is also a local site investigator on a Department of Veterans Affairs sponsored multi-site trial of treatments for resistant depression. One study arm is receiving aripiprazole augmentation. The study is receiving no funding from pharmaceutical companies. NZR is also serving as a consultant in a lawsuit against the manufacturer of aripiprazole. ACT acknowledges salary support through Mentored Patient-Oriented Research Career Development Award K23 MH-096620, US National Institute of Mental Health. ACT is a former board member of the ethics committee, and former member, of the National Physicians Alliance. GIS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. No specific funding was provided to support the conduct of this meta-analysis. The authors declare no other competing interests.
Figures


References
-
- Sigal E (2009) Bristol-Myers Squibb Company Q1 2009 earnings call transcript. Seeking Alpha Available: http://seekingalpha.com/article/133733-bristol-myers-squibb-company-q1-2.... Accessed 7 May 2009.
-
- Olfson M, Marcus SC (2009) National patterns in antidepressant medication treatment. Arch Gen Psychiatry 66: 848–856. - PubMed
-
- Leslie DL, Mohamed S, Rosenheck RA (2009) Off-label use of antipsychotic medications in the department of Veterans Affairs health care system. Psychiatr Serv 60: 1175–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

