Flotillin-2 is an acrosome-related protein involved in mouse spermiogenesis
- PMID: 23554761
- PMCID: PMC3596745
- DOI: 10.7555/JBR.26.20120030
Flotillin-2 is an acrosome-related protein involved in mouse spermiogenesis
Abstract
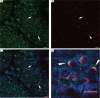
SPERMATOGENESIS IS A COMPLEX PROCESS OF TERMINAL DIFFERENTIATION BY WHICH MATURE SPERMS ARE GENERATED, AND IT CAN BE DIVIDED INTO THREE PHASES: mitosis, meiosis and spermiogenesis. In a previous study, we established a series of proteomic profiles for spermatogenesis to understand the regulation of male fertility and infertility. Here, we further investigated the localization and the role of flotillin-2 in spermiogenesis. Flotillin-2 expression was investigated in the testis of male CD1 mice at various developmental stages of spermatogenesis by using Western blotting, immunohistochemistry and immunofluorescence. Flotillin-2 was knocked down in vivo in three-week-old male mice using intratesticular injection of small inhibitory RNA (siRNA), and sperm abnormalities were assessed three weeks later. Flotillin-2 was expressed at high levels in male germ cells during spermatogenesis. Flotillin-2 immunoreactivity was observed in pachytene spermatocytes as a strong dot-shaped signal and in round spermatids as a sickle-shaped distribution ahead of the acrosome. Immunofluorescence confirmed flotillin-2 was localized in front of the acrosome in round spermatids, indicating that flotillin-2 was localized to the Golgi apparatus. Knockdown of flotillin-2 in vivo led to a significant increase in head sperm abnormalities isolated from the cauda epididymis, compared with control siRNA-injected testes. This study indicates that flotillin-2 is a novel Golgi-related protein involved in sperm acrosome biogenesis.
Keywords: Golgi apparatus; RNA interference; acrosome biogenesis; flotillin-2.
Conflict of interest statement
The authors reported no conflict of interest.
Figures






Similar articles
-
Expression of Flotilin-2 and Acrosome Biogenesis Are Regulated by MiR-124 during Spermatogenesis.PLoS One. 2015 Aug 27;10(8):e0136671. doi: 10.1371/journal.pone.0136671. eCollection 2015. PLoS One. 2015. PMID: 26313572 Free PMC article.
-
Lamin A/C proteins in the spermatid acroplaxome are essential in mouse spermiogenesis.Reproduction. 2014 Nov;148(5):479-87. doi: 10.1530/REP-14-0012. Epub 2014 Aug 12. Reproduction. 2014. PMID: 25118303
-
Seipin deficiency increases chromocenter fragmentation and disrupts acrosome formation leading to male infertility.Cell Death Dis. 2015 Jul 16;6(7):e1817. doi: 10.1038/cddis.2015.188. Cell Death Dis. 2015. PMID: 26181198 Free PMC article.
-
[Sperm acrosome formation-associated genes in mice: Advances in studies].Zhonghua Nan Ke Xue. 2016 Jan;22(1):72-6. Zhonghua Nan Ke Xue. 2016. PMID: 26931031 Review. Chinese.
-
Organization and modifications of sperm acrosomal molecules during spermatogenesis and epididymal maturation.Microsc Res Tech. 2003 May 1;61(1):39-45. doi: 10.1002/jemt.10315. Microsc Res Tech. 2003. PMID: 12672121 Review.
Cited by
-
TDRP deficiency contributes to low sperm motility and is a potential risk factor for male infertility.Am J Transl Res. 2016 Jan 15;8(1):177-87. eCollection 2016. Am J Transl Res. 2016. PMID: 27069551 Free PMC article.
-
The ontogenic gonadal transcriptomes provide insights into sex change in the ricefield eel Monopterus albus.BMC Zool. 2022 Nov 23;7(1):56. doi: 10.1186/s40850-022-00155-4. BMC Zool. 2022. PMID: 37170354 Free PMC article.
-
Proteogenomics of Gammarus fossarum to document the reproductive system of amphipods.Mol Cell Proteomics. 2014 Dec;13(12):3612-25. doi: 10.1074/mcp.M114.038851. Epub 2014 Oct 7. Mol Cell Proteomics. 2014. PMID: 25293947 Free PMC article.
-
The control of male fertility by spermatid-specific factors: searching for contraceptive targets from spermatozoon's head to tail.Cell Death Dis. 2016 Nov 10;7(11):e2472. doi: 10.1038/cddis.2016.344. Cell Death Dis. 2016. PMID: 27831554 Free PMC article. Review.
-
Expression of Flotilin-2 and Acrosome Biogenesis Are Regulated by MiR-124 during Spermatogenesis.PLoS One. 2015 Aug 27;10(8):e0136671. doi: 10.1371/journal.pone.0136671. eCollection 2015. PLoS One. 2015. PMID: 26313572 Free PMC article.
References
-
- Ramalho-Santos J, Schatten G, Moreno RD. Control of membrane fusion during spermiogenesis and the acrosome reaction. Biol Reprod. 2002;67:1043–51. - PubMed
-
- Moreno RD, Ramalho-Santos J, Chan EK, Wessel GM, Schatten G. The Golgi apparatus segregates from the lysosomal/acrosomal vesicle during rhesus spermiogenesis: structural alterations. Dev Biol. 2000;219:334–49. - PubMed
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech. 2010;73:279–319. - PubMed
-
- Ramalho-Santos J, Moreno RD, Wessel GM, Chan EK, Schatten G. Membrane trafficking machinery components associated with the mammalian acrosome during spermiogenesis. Exp Cell Res. 2001;267:45–60. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

