Lipoprotein metabolism in nonalcoholic fatty liver disease
- PMID: 23554788
- PMCID: PMC3596749
- DOI: 10.7555/JBR.27.20120077
Lipoprotein metabolism in nonalcoholic fatty liver disease
Abstract
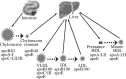
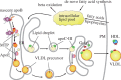
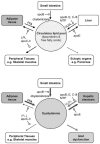
Nonalcoholic fatty liver disease (NAFLD), an escalating health problem worldwide, covers a spectrum of pathologies characterized by fatty accumulation in hepatocytes in early stages, with potential progression to liver inflammation, fibrosis, and failure. A close, yet poorly understood link exists between NAFLD and dyslipidemia, a constellation of abnormalities in plasma lipoproteins including triglyceride-rich very low density lipoproteins. Apolipoproteins are a group of primarily liver-derived proteins found in serum lipoproteins; they not only play an extracellular role in lipid transport between vital organs through circulation, but also play an important intracellular role in hepatic lipoprotein assembly and secretion. The liver functions as the central hub for lipoprotein metabolism, as it dictates lipoprotein production and to a significant extent modulates lipoprotein clearance. Lipoprotein metabolism is an integral component of hepatocellular lipid homeostasis and is implicated in the pathogenesis, potential diagnosis, and treatment of NAFLD.
Keywords: apolipoprotein; hepatic steatosis; lipoprotein metabolism; nonalcoholic fatty liver disease (NAFLD); nonalcoholic steatohepatitis; very low density lipoprotein.
Conflict of interest statement
The authors reported no conflict of interest.
Figures

Similar articles
-
Hepatic Steatosis and Insulin Resistance, But Not Steatohepatitis, Promote Atherogenic Dyslipidemia in NAFLD.J Clin Endocrinol Metab. 2016 Feb;101(2):644-52. doi: 10.1210/jc.2015-3111. Epub 2015 Dec 16. J Clin Endocrinol Metab. 2016. PMID: 26672634
-
Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease.World J Gastroenterol. 2019 Jun 28;25(24):3009-3020. doi: 10.3748/wjg.v25.i24.3009. World J Gastroenterol. 2019. PMID: 31293337 Free PMC article. Review.
-
The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease.Children (Basel). 2017 Jun 6;4(6):46. doi: 10.3390/children4060046. Children (Basel). 2017. PMID: 28587303 Free PMC article. Review.
-
IMPACT OF TYPE 2 DIABETES ON NONALCOHOLIC STEATOHEPATITIS AND ADVANCED FIBROSIS IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE.Endocr Pract. 2020 Apr;26(4):444-453. doi: 10.4158/EP-2019-0342. Epub 2020 Jan 22. Endocr Pract. 2020. PMID: 31968197
-
Genetic Determinants of Circulating Lipoproteins in Nonalcoholic Fatty Liver Disease.J Clin Gastroenterol. 2018 May/Jun;52(5):444-451. doi: 10.1097/MCG.0000000000000816. J Clin Gastroenterol. 2018. PMID: 28362682 Free PMC article.
Cited by
-
Role of PCSK9 inhibition during the inflammatory stage of SARS-COV-2: an updated review.Ann Med Surg (Lond). 2024 Jan 3;86(2):899-908. doi: 10.1097/MS9.0000000000001601. eCollection 2024 Feb. Ann Med Surg (Lond). 2024. PMID: 38333263 Free PMC article. Review.
-
Sodium fluorocitrate having inhibitory effect on fatty acid uptake ameliorates high fat diet-induced non-alcoholic fatty liver disease in C57BL/6J mice.Sci Rep. 2019 Nov 28;9(1):17839. doi: 10.1038/s41598-019-54476-5. Sci Rep. 2019. PMID: 31780766 Free PMC article.
-
Differential genomic effects of four nano-sized and one micro-sized CeO2 particles on HepG2 cells.Mater Express. 2023 Oct;13(10):1799-1811. doi: 10.1166/mex.2023.2527. Mater Express. 2023. PMID: 38009104 Free PMC article.
-
Steatohepatitis and liver fibrosis are predicted by the characteristics of very low density lipoprotein in nonalcoholic fatty liver disease.Liver Int. 2016 Aug;36(8):1213-20. doi: 10.1111/liv.13076. Epub 2016 Feb 24. Liver Int. 2016. PMID: 26815314 Free PMC article.
-
Non-alcoholic fatty liver disease--a chronic disease of the 21st century.J Biomed Res. 2018 Sep 29;32(5):327-335. doi: 10.7555/JBR.31.20160153. J Biomed Res. 2018. PMID: 28550272 Free PMC article.
References
-
- Davidson B, Cliff G. Liver lipids of female Carcharias taurus (spotted raggedtooth) sharks: a comparison between seasons. Fish Physiol Biochem. 2011;37:613–8. - PubMed
-
- Kaplan MM. Acute fatty liver of pregnancy. N Engl J Med. 1985;313:367–70. - PubMed
-
- Lieber CS, Rubin E. Alcoholic fatty liver. N Engl J Med. 1969;280:705–8. - PubMed
-
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. - PubMed
-
- Brunt EM. Non-alcoholic fatty liver disease: what's new under the microscope? Gut. 2011;60:1152–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

