Comparison of PfHRP-2/pLDH ELISA, qPCR and microscopy for the detection of plasmodium events and prediction of sick visits during a malaria vaccine study
- PMID: 23554856
- PMCID: PMC3598859
- DOI: 10.1371/journal.pone.0056828
Comparison of PfHRP-2/pLDH ELISA, qPCR and microscopy for the detection of plasmodium events and prediction of sick visits during a malaria vaccine study
Abstract
Background: Compared to expert malaria microscopy, malaria biomarkers such as Plasmodium falciparum histidine rich protein-2 (PfHRP-2), and PCR provide superior analytical sensitivity and specificity for quantifying malaria parasites infections. This study reports on parasite prevalence, sick visits parasite density and species composition by different diagnostic methods during a phase-I malaria vaccine trial.
Methods: Blood samples for microscopy, PfHRP-2 and Plasmodium lactate dehydrogenase (pLDH) ELISAs and real time quantitative PCR (qPCR) were collected during scheduled (n = 298) or sick visits (n = 38) from 30 adults participating in a 112-day vaccine trial. The four methods were used to assess parasite prevalence, as well as parasite density over a 42-day period for patients with clinical episodes.
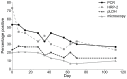
Results: During scheduled visits, qPCR (39.9%, N = 119) and PfHRP-2 ELISA (36.9%, N = 110) detected higher parasite prevalence than pLDH ELISA (16.8%, N = 50) and all methods were more sensitive than microscopy (13.4%, N = 40). All microscopically detected infections contained P. falciparum, as mono-infections (95%) or with P. malariae (5%). By qPCR, 102/119 infections were speciated. P. falciparum predominated either as monoinfections (71.6%), with P. malariae (8.8%), P. ovale (4.9%) or both (3.9%). P. malariae (6.9%) and P. ovale (1.0%) also occurred as co-infections (2.9%). As expected, higher prevalences were detected during sick visits, with prevalences of 65.8% (qPCR), 60.5% (PfHRP-2 ELISA), 21.1% (pLDH ELISA) and 31.6% (microscopy). PfHRP-2 showed biomass build-up that climaxed (1813±3410 ng/mL SD) at clinical episodes.
Conclusion: PfHRP-2 ELISA and qPCR may be needed for accurately quantifying the malaria parasite burden. In addition, qPCR improves parasite speciation, whilst PfHRP-2 ELISA is a potential predictor for clinical disease caused by P. falciparum.
Trial registration: ClinicalTrials.gov NCT00666380.
Conflict of interest statement
Figures

References
-
- World Health Organization (2008) Global malaria control and elimination: report of a technical review. (http://www.whqlibdoc.who.int/publications/2008/9789241596756_eng.pdf).
-
- Okell LC, Ghani AC, Lyons E, Drakeley CJ (2009) Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 200 (10) 1509–17. - PubMed
-
- Guerin PJ, Olliaro P, Nosten F, Druilhe P, Laxminarayan R, et al. (2002) Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect Dis 2 (9) 564–573. - PubMed
-
- Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, et al. (2011) Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of Plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol 49 (8) 2946–2953. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

