Functional implications of microRNA-215 in TGF-β1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1
- PMID: 23554908
- PMCID: PMC3595285
- DOI: 10.1371/journal.pone.0058622
Functional implications of microRNA-215 in TGF-β1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1
Abstract
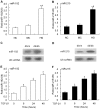
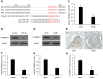
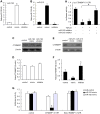
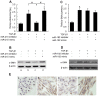
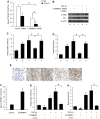
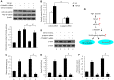
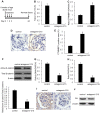
Mesangial cell (MC) phenotypic transition is crucial for the progression of diabetic nephropathy. A major stimulus mediating high glucose-induced MC phenotypic transition is TGF-β1. Our current study focuses on microRNA-215 (miR-215) and investigates its role in TGF-β1-mediated MC phenotypic transition. Using real-time quantitative PCR (qRT-PCR) and northern blotting, we determined that the miR-192/215 family is dramatically upregulated under diabetic conditions both in vitro and in vivo. Gain- and loss-of-function approaches demonstrated that miR-215 inhibition significantly inhibited TGF-β1-induced mouse mesangial cell (MMC) phenotypic transition, whereas miR-215 upregulation promoted MMC phenotypic transition. Interestingly, these changes were not detected in cells that were treated with TGF-β1 and miR-192 mimics or inhibitors. These results suggest that miR-215 participates in TGF-β1-induced MMC phenotypic transition. Luciferase reporter assays were used to identify whether catenin-beta interacting protein 1 (CTNNBIP1) is a direct target of miR-215, which was predicted by bioinformatic analysis. Mechanistic studies revealed that CTNNBIP1 suppresses Wnt/β-catenin signaling and that miR-215 promotes β-catenin activation and upregulates α-SMA and fibronectin expression in TGF-β1-treated MMCs by targeting CTNNBIP1. In addition, in vivo miR-215 silencing with a specific antagomir significantly increased CTNNBIP1 protein expression, resulting in reduced β-catenin activity and decreased α-SMA and fibronectin expression in db/db mouse kidney glomeruli. Taken together, our findings indicate that miR-215 plays an essential role in MC phenotypic transition by regulating the CTNNBIP1/β-catenin pathway, which is related to the pathogenesis of diabetic nephropathy.
Conflict of interest statement
Figures
References
-
- Balakumar P, Arora MK, Reddy J, Anand-Srivastava MB (2009) Pathophysiology of diabetic nephropathy: involvement of multifaceted signalling mechanism. J Cardiovasc Pharmacol 54: 129–138. - PubMed
-
- Fogo AB (1999) Mesangial matrix modulation and glomerulosclerosis. Exp Nephrol 7: 147–159. - PubMed
-
- Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, et al. (1997) Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant 12: 43–50. - PubMed
-
- Simonson MS (2007) Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int 71: 846–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

