Up-regulation of intestinal epithelial cell derived IL-7 expression by keratinocyte growth factor through STAT1/IRF-1, IRF-2 pathway
- PMID: 23554911
- PMCID: PMC3595257
- DOI: 10.1371/journal.pone.0058647
Up-regulation of intestinal epithelial cell derived IL-7 expression by keratinocyte growth factor through STAT1/IRF-1, IRF-2 pathway
Retraction in
-
Retraction: Up-Regulation of Intestinal Epithelial Cell Derived IL-7 Expression by Keratinocyte Growth Factor through STAT1/IRF-1, IRF-2 Pathway.PLoS One. 2024 Jun 18;19(6):e0305938. doi: 10.1371/journal.pone.0305938. eCollection 2024. PLoS One. 2024. PMID: 38889137 Free PMC article. No abstract available.
Abstract
Background: Epithelial cells(EC)-derived interleukin-7 (IL-7) plays a crucial role in control of development and homeostasis of neighboring intraepithelial lymphocytes (IEL), and keratinocyte growth factor (KGF) exerts protective effects on intestinal epithelial cells and up-regulates EC-derived IL-7 expression through KGFR pathway. This study was to further investigate the molecular mechanism involved in the regulation of IL-7 expression by KGF in the intestine.
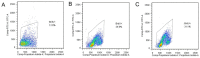
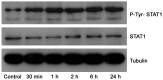
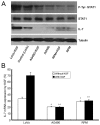
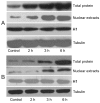
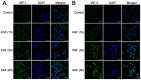
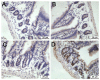
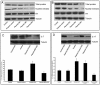
Methods: Intestinal epithelial cells (LoVo cells) and adult C57BL/6J mice were treated with KGF. Epithelial cell proliferation was studied by flow cytometry for BrdU-incorporation and by immunohistochemistry for PCNA staining. Western blot was used to detect the changes of expression of P-Tyr-STAT1, STAT1, and IL-7 by inhibiting STAT1. Alterations of nuclear extracts and total proteins of IRF-1, IRF-2 and IL-7 following IRF-1 and IRF-2 RNA interference with KGF treatment were also measured with western blot. Moreover, IL-7 mRNA expressions were also detected by Real-time PCR and IL-7 protein level in culture supernatants was measured by enzyme linked immunosorbent assay(ELISA).
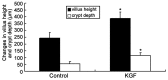
Results: KGF administration significantly increased LoVo cell proliferation and also increased intestinal wet weight, villus height, crypt depth and crypt cell proliferation in mice. KGF treatment led to increased levels of P-Tyr-STAT1, RAPA and AG490 both blocked P-Tyr-STAT1 and IL-7 expression in LoVo cells. IRF-1 and IRF-2 expression in vivo and in vitro were also up-regulated by KGF, and IL-7 expression was decreased after IRF-1 and IRF-2 expression was silenced by interfering RNA, respectively.
Conclusion: KGF could up-regulate IL-7 expression through the STAT1/IRF-1, IRF-2 signaling pathway, which is a new insight in potential effects of KGF on the intestinal mucosal immune system.
Conflict of interest statement
Figures

Similar articles
-
Keratinocyte growth factor up-regulates Interleukin-7 expression following intestinal ischemia/reperfusion in vitro and in vivo.Int J Clin Exp Pathol. 2012;5(6):569-80. Epub 2012 Jul 29. Int J Clin Exp Pathol. 2012. PMID: 22949940 Free PMC article.
-
2002 Harry M. Vars Research Award. Keratinocyte growth factor stimulates the recovery of epithelial structure and function in a mouse model of total parenteral nutrition.JPEN J Parenter Enteral Nutr. 2002 Nov-Dec;26(6):333-40; discussion 340-1. doi: 10.1177/0148607102026006333. JPEN J Parenter Enteral Nutr. 2002. PMID: 12405644
-
AhR‑E2F1‑KGFR signaling is involved in KGF‑induced intestinal epithelial cell proliferation.Mol Med Rep. 2017 May;15(5):3019-3026. doi: 10.3892/mmr.2017.6368. Epub 2017 Mar 23. Mol Med Rep. 2017. PMID: 28339052 Free PMC article.
-
Keratinocyte growth factor pretreatment prevents radiation-induced intestinal damage in a mouse model.Scand J Gastroenterol. 2013 Apr;48(4):419-26. doi: 10.3109/00365521.2013.772227. Epub 2013 Mar 7. Scand J Gastroenterol. 2013. PMID: 23464848
-
The effects of keratinocyte growth factor in preclinical models of mucositis.Cell Prolif. 2002 Aug;35 Suppl 1(Suppl 1):78-85. doi: 10.1046/j.1365-2184.35.s1.8.x. Cell Prolif. 2002. PMID: 12139710 Free PMC article. Review.
Cited by
-
Fibroblast growth factor 10 alters the balance between goblet and Paneth cells in the adult mouse small intestine.Am J Physiol Gastrointest Liver Physiol. 2015 Apr 15;308(8):G678-90. doi: 10.1152/ajpgi.00158.2014. Epub 2015 Feb 26. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25721301 Free PMC article.
-
Interleukin (IL)-7 Signaling in the Tumor Microenvironment.Adv Exp Med Biol. 2021;1290:9-49. doi: 10.1007/978-3-030-55617-4_2. Adv Exp Med Biol. 2021. PMID: 33559853
-
Expression of interferon regulatory factors (IRF-1 and IRF-2) during radiation-induced damage and regeneration of bone marrow by transplantation in mouse.Mol Biol Rep. 2019 Feb;46(1):551-567. doi: 10.1007/s11033-018-4508-x. Epub 2018 Nov 28. Mol Biol Rep. 2019. PMID: 30488374
-
Role of FGF10/FGFR2b Signaling in Mouse Digestive Tract Development, Repair and Regeneration Following Injury.Front Cell Dev Biol. 2019 Dec 10;7:326. doi: 10.3389/fcell.2019.00326. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31921841 Free PMC article. Review.
-
Repression of SUMOylation pathway by grass carp reovirus contributes to the upregulation of PKR in an IFN-independent manner.Oncotarget. 2017 Aug 17;8(42):71500-71511. doi: 10.18632/oncotarget.20309. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 29069722 Free PMC article.
References
-
- Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J, et al. (2001) IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol 166 (5) 3019–27. - PubMed
-
- Bilenker M, Roberts AI, Brolin RE, Ebert EC (1995) Interleukin-7 activates intestinal lymphocytes. Dig Dis Sci 40 (8) 1744–1749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

