Noninvasive ultrasound molecular imaging of the effect of statins on endothelial inflammatory phenotype in early atherosclerosis
- PMID: 23554922
- PMCID: PMC3598944
- DOI: 10.1371/journal.pone.0058761
Noninvasive ultrasound molecular imaging of the effect of statins on endothelial inflammatory phenotype in early atherosclerosis
Abstract
Background/objectives: Inflammatory changes on the endothelium are responsible for leukocyte recruitment to plaques in atherosclerosis. Noninvasive assessment of treatment-effects on endothelial inflammation may be of use for managing medical therapy and developing novel therapies. We hypothesized that molecular imaging of vascular cell adhesion molecule-1 (VCAM-1) with contrast enhanced ultrasound (CEU) could assess treatment effects on endothelial phenotype in early atherosclerosis.
Methods: Mice with atherosclerosis produced by gene deletion of the LDL-receptor and Apobec-1-editing protein were studied. At 12 weeks of age, mice received 8 weeks of regular chow or atorvastatin-enriched chow (10 mg/kg/day). At 20 weeks, CEU molecular imaging for aortic endothelial VCAM-1 expression was performed with VCAM-1-targeted (MB(VCAM)) and control microbubbles (MB(Ctr)). Aortic wall thickness was assessed with high frequency ultrasound. Histology, immunohistology and Western blot were used to assess plaque burden and VCAM-1 expression.
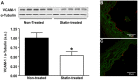
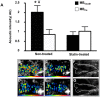
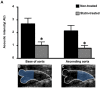
Results: Plaque burden was reduced on histology, and VCAM-1 was reduced on Western blot by atorvastatin, which corresponded to less endothelial expression of VCAM-1 on immunohistology. High frequency ultrasound did not detect differences in aortic wall thickness between groups. In contrast, CEU molecular imaging demonstrated selective signal enhancement for MB(VCAM) in non-treated animals (MB(VCAM) 2±0.3 vs MB(Ctr) 0.7±0.2, p<0.01), but not in statin-treated animals (MB(VCAM) 0.8±0.2 vs MB(Ctr) 1.0±0.2, p = ns; p<0.01 for the effect of statin on MB(VCAM) signal).
Conclusions: Non-invasive CEU molecular imaging detects the effects of anti-inflammatory treatment on endothelial inflammation in early atherosclerosis. This easily accessible, low-cost technique may be useful in assessing treatment effects in preclinical research and in patients.
Conflict of interest statement
Figures



References
-
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, et al. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of scotland coronary prevention study group. N Engl J Med 333: 1301–1307. - PubMed
-
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of afcaps/texcaps. Air force/texas coronary atherosclerosis prevention study. JAMA 279: 1615–1622. - PubMed
-
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, et al. (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the anglo-scandinavian cardiac outcomes trial–lipid lowering arm (ascot-lla): A multicentre randomised controlled trial. Lancet 361: 1149–1158. - PubMed
-
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, et al. (2008) Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med 359: 2195–2207. - PubMed
-
- Chyu KY, Shah PK (2011) Emerging therapies for atherosclerosis prevention and management. Cardiol Clin 29: 123–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

