Tooth eruption results from bone remodelling driven by bite forces sensed by soft tissue dental follicles: a finite element analysis
- PMID: 23554928
- PMCID: PMC3598949
- DOI: 10.1371/journal.pone.0058803
Tooth eruption results from bone remodelling driven by bite forces sensed by soft tissue dental follicles: a finite element analysis
Abstract
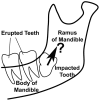
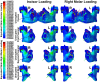
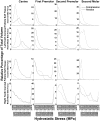
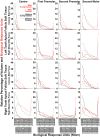
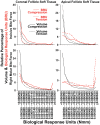
Intermittent tongue, lip and cheek forces influence precise tooth position, so we here examine the possibility that tissue remodelling driven by functional bite-force-induced jaw-strain accounts for tooth eruption. Notably, although a separate true 'eruptive force' is widely assumed, there is little direct evidence for such a force. We constructed a three dimensional finite element model from axial computerized tomography of an 8 year old child mandible containing 12 erupted and 8 unerupted teeth. Tissues modelled included: cortical bone, cancellous bone, soft tissue dental follicle, periodontal ligament, enamel, dentine, pulp and articular cartilage. Strain and hydrostatic stress during incisive and unilateral molar bite force were modelled, with force applied via medial and lateral pterygoid, temporalis, masseter and digastric muscles. Strain was maximal in the soft tissue follicle as opposed to surrounding bone, consistent with follicle as an effective mechanosensor. Initial numerical analysis of dental follicle soft tissue overlying crowns and beneath the roots of unerupted teeth was of volume and hydrostatic stress. To numerically evaluate biological significance of differing hydrostatic stress levels normalized for variable finite element volume, 'biological response units' in Nmm were defined and calculated by multiplication of hydrostatic stress and volume for each finite element. Graphical representations revealed similar overall responses for individual teeth regardless if incisive or right molar bite force was studied. There was general compression in the soft tissues over crowns of most unerupted teeth, and general tension in the soft tissues beneath roots. Not conforming to this pattern were the unerupted second molars, which do not erupt at this developmental stage. Data support a new hypothesis for tooth eruption, in which the follicular soft tissues detect bite-force-induced bone-strain, and direct bone remodelling at the inner surface of the surrounding bony crypt, with the effect of enabling tooth eruption into the mouth.
Conflict of interest statement
Figures








Similar articles
-
The effect of removing the true dental follicle on premolar eruption in the dog.Arch Oral Biol. 1994 Apr;39(4):271-5. doi: 10.1016/0003-9969(94)90116-3. Arch Oral Biol. 1994. PMID: 8024490
-
Finite element analysis suggests functional bone strain accounts for continuous post-eruptive emergence of teeth.Arch Oral Biol. 2012 Aug;57(8):1070-8. doi: 10.1016/j.archoralbio.2012.05.001. Epub 2012 Jun 5. Arch Oral Biol. 2012. PMID: 22673755
-
Biomechanical investigation into the role of the periodontal ligament in optimising orthodontic force: a finite element case study.Arch Oral Biol. 2016 Jun;66:98-107. doi: 10.1016/j.archoralbio.2016.02.012. Epub 2016 Feb 23. Arch Oral Biol. 2016. PMID: 26943815
-
Current concepts of the biology of tooth eruption.Crit Rev Oral Biol Med. 1992;3(3):185-206. doi: 10.1177/10454411920030030201. Crit Rev Oral Biol Med. 1992. PMID: 1571471 Review.
-
Tooth Root Development and Homeostasis during Eruptive and Post-eruptive Movement.Chin J Dent Res. 2024 Dec 6;27(4):273-289. doi: 10.3290/j.cjdr.b5860254. Chin J Dent Res. 2024. PMID: 39641291 Review.
Cited by
-
Biomechanics of oral mucosa.J R Soc Interface. 2015 Aug 6;12(109):20150325. doi: 10.1098/rsif.2015.0325. J R Soc Interface. 2015. PMID: 26224566 Free PMC article. Review.
-
Resorptive potential of impacted mandibular third molars: 3D simulation by finite element analysis.Clin Oral Investig. 2018 Dec;22(9):3195-3203. doi: 10.1007/s00784-018-2403-4. Epub 2018 Mar 10. Clin Oral Investig. 2018. PMID: 29525922
-
Three-dimensional dentoalveolar characteristics of a labially impacted dilacerated maxillary central incisor using cone-beam computed tomography.Sci Rep. 2025 Jul 9;15(1):24669. doi: 10.1038/s41598-025-10043-9. Sci Rep. 2025. PMID: 40634539 Free PMC article.
-
A three-dimensional finite element analysis of molar distalization with a palatal plate, pendulum, and headgear according to molar eruption stage.Korean J Orthod. 2016 Sep;46(5):290-300. doi: 10.4041/kjod.2016.46.5.290. Epub 2016 Sep 19. Korean J Orthod. 2016. PMID: 27668192 Free PMC article.
-
Is there a relationship between the presence of external root resorption in second molars adjacent to impacted mandibular third molars with awake bruxism and masticatory muscle activity?Clin Oral Investig. 2024 Jun 28;28(7):401. doi: 10.1007/s00784-024-05775-3. Clin Oral Investig. 2024. PMID: 38940883
References
-
- Avery JK, Steele PF, Avery N (2001) Oral Development and Histology. Stuttgart: Thieme.
-
- Proffit WR (1978) Equilibrium theory revisited: factors influencing position of the teeth. Angle Orthod 48: 175–186. - PubMed
-
- Steedle JR, Proffit WR (1985) The pattern and control of eruptive tooth movements. Am J Orthod 87: 56–66. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

