Zebrafish hoxd4a acts upstream of meis1.1 to direct vasculogenesis, angiogenesis and hematopoiesis
- PMID: 23554940
- PMCID: PMC3598951
- DOI: 10.1371/journal.pone.0058857
Zebrafish hoxd4a acts upstream of meis1.1 to direct vasculogenesis, angiogenesis and hematopoiesis
Abstract
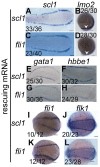
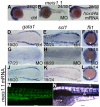
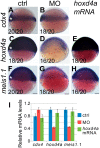
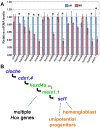
Mice lacking the 4th-group paralog Hoxd4 display malformations of the anterior vertebral column, but are viable and fertile. Here, we report that zebrafish embryos having decreased function of the orthologous hoxd4a gene manifest striking perturbations in vasculogenesis, angiogenesis and primitive and definitive hematopoiesis. These defects are preceded by reduced expression of the hemangioblast markers scl1, lmo2 and fli1 within the posterior lateral plate mesoderm (PLM) at 13 hours post fertilization (hpf). Epistasis analysis revealed that hoxd4a acts upstream of meis1.1 but downstream of cdx4 as early as the shield stage in ventral-most mesoderm fated to give rise to hemangioblasts, leading us to propose that loss of hoxd4a function disrupts hemangioblast specification. These findings place hoxd4a high in a genetic hierarchy directing hemangioblast formation downstream of cdx1/cdx4 and upstream of meis1.1. An additional consequence of impaired hoxd4a and meis1.1 expression is the deregulation of multiple Hox genes implicated in vasculogenesis and hematopoiesis which may further contribute to the defects described here. Our results add to evidence implicating key roles for Hox genes in their initial phase of expression early in gastrulation.
Conflict of interest statement
Figures


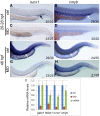
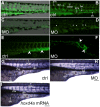
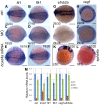
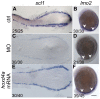
Similar articles
-
A zebrafish hox gene acts before gastrulation to specify the hemangioblast.Genesis. 2020 Jun;58(6):e23363. doi: 10.1002/dvg.23363. Epub 2020 Apr 17. Genesis. 2020. PMID: 32302038
-
The Hox cofactors Meis1 and Pbx act upstream of gata1 to regulate primitive hematopoiesis.Dev Biol. 2010 Apr 15;340(2):306-17. doi: 10.1016/j.ydbio.2010.01.033. Epub 2010 Feb 1. Dev Biol. 2010. PMID: 20123093
-
MiR-144 regulates hematopoiesis and vascular development by targeting meis1 during zebrafish development.Int J Biochem Cell Biol. 2014 Apr;49:53-63. doi: 10.1016/j.biocel.2014.01.005. Epub 2014 Jan 18. Int J Biochem Cell Biol. 2014. PMID: 24448023
-
Identification of a new adtrp1-tfpi regulatory axis for the specification of primitive myelopoiesis and definitive hematopoiesis.FASEB J. 2018 Jan;32(1):183-194. doi: 10.1096/fj.201700166RR. Epub 2017 Sep 6. FASEB J. 2018. PMID: 28877957 Free PMC article.
-
Aggf1 acts at the top of the genetic regulatory hierarchy in specification of hemangioblasts in zebrafish.Blood. 2014 Jan 23;123(4):501-8. doi: 10.1182/blood-2013-07-514612. Epub 2013 Nov 25. Blood. 2014. PMID: 24277077 Free PMC article.
Cited by
-
Testing biological actions of medicinal plants from northern Vietnam on zebrafish embryos and larvae: Developmental, behavioral, and putative therapeutical effects.PLoS One. 2023 Nov 7;18(11):e0294048. doi: 10.1371/journal.pone.0294048. eCollection 2023. PLoS One. 2023. PMID: 37934745 Free PMC article.
-
Hox proteins as regulators of extracellular matrix interactions during neural crest migration.Differentiation. 2022 Nov-Dec;128:26-32. doi: 10.1016/j.diff.2022.09.003. Epub 2022 Oct 4. Differentiation. 2022. PMID: 36228422 Free PMC article. Review.
-
Homeobox gene Meis1 modulates cardiovascular regeneration.Semin Cell Dev Biol. 2020 Apr;100:52-61. doi: 10.1016/j.semcdb.2019.10.003. Epub 2019 Oct 14. Semin Cell Dev Biol. 2020. PMID: 31623926 Free PMC article. Review.
-
Origin and differentiation of vascular smooth muscle cells.J Physiol. 2015 Jul 15;593(14):3013-30. doi: 10.1113/JP270033. Epub 2015 Jun 9. J Physiol. 2015. PMID: 25952975 Free PMC article. Review.
-
Kindlin-2 deficiency induces fatal intestinal obstruction in mice.Theranostics. 2020 May 15;10(14):6182-6200. doi: 10.7150/thno.46553. eCollection 2020. Theranostics. 2020. PMID: 32483447 Free PMC article.
References
-
- Paik EJ, Zon LI (2010) Hematopoietic development in the zebrafish. Int J Dev Biol 54: 1127–1137. - PubMed
-
- Ellertsdottir E, Lenard A, Blum Y, Krudewig A, Herwig L, et al. (2010) Vascular morphogenesis in the zebrafish embryo. Dev Biol 341: 56–65. - PubMed
-
- Ciau-Uitz A, Liu F, Patient R (2010) Genetic control of hematopoietic development in Xenopus and zebrafish. Int J Dev Biol 54: 1139–1149. - PubMed
-
- Chen AT, Zon LI (2009) Zebrafish blood stem cells. J Cell Biochem 108: 35–42. - PubMed
-
- Vogeli KM, Jin SW, Martin GR, Stainier DY (2006) A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 443: 337–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

