MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation
- PMID: 23554954
- PMCID: PMC3595229
- DOI: 10.1371/journal.pone.0058897
MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation
Abstract
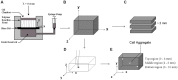
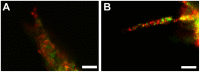
The spatial presentation of immobilized extracellular matrix (ECM) cues and matrix mechanical properties play an important role in directed and guided cell behavior and neovascularization. The goal of this work was to explore whether gradients of elastic modulus, immobilized matrix metalloproteinase (MMP)-sensitivity, and YRGDS cell adhesion ligands are capable of directing 3D vascular sprout formation in tissue engineered scaffolds. PEGDA hydrogels were engineered with mechanical and biofunctional gradients using perfusion-based frontal photopolymerization (PBFP). Bulk photopolymerized hydrogels with uniform mechanical properties, degradation, and immobilized biofunctionality served as controls. Gradient hydrogels exhibited an 80.4% decrease in elastic modulus and a 56.2% decrease in immobilized YRGDS. PBFP hydrogels also demonstrated gradients in hydrogel degradation with degradation times ranging from 10-12 hours in the more crosslinked regions to 4-6 hours in less crosslinked regions. An in vitro model of neovascularization, composed of co-culture aggregates of endothelial and smooth muscle cells, was used to evaluate the effect of these gradients on vascular sprout formation. Aggregate invasion in gradient hydrogels occurred bi-directionally with sprout alignment observed in the direction parallel to the gradient while control hydrogels with homogeneous properties resulted in uniform invasion. In PBFP gradient hydrogels, aggregate sprout length was found to be twice as long in the direction parallel to the gradient as compared to the perpendicular direction after three weeks in culture. This directionality was found to be more prominent in gradient regions of increased stiffness, crosslinked MMP-sensitive peptide presentation, and immobilized YRGDS concentration.
Conflict of interest statement
Figures







Similar articles
-
Generation of mechanical and biofunctional gradients in PEG diacrylate hydrogels by perfusion-based frontal photopolymerization.J Biomater Sci Polym Ed. 2012;23(7):917-39. doi: 10.1163/092050611X566450. J Biomater Sci Polym Ed. 2012. PMID: 21477459
-
Controlled proteolytic cleavage site presentation in biomimetic PEGDA hydrogels enhances neovascularization in vitro.Tissue Eng Part A. 2012 Dec;18(23-24):2477-86. doi: 10.1089/ten.TEA.2012.0173. Epub 2012 Jul 25. Tissue Eng Part A. 2012. PMID: 22725267 Free PMC article.
-
Protease-Sensitive Hydrogel Biomaterials with Tunable Modulus and Adhesion Ligand Gradients for 3D Vascular Sprouting.Biomacromolecules. 2018 Nov 12;19(11):4168-4181. doi: 10.1021/acs.biomac.8b00519. Epub 2018 Oct 15. Biomacromolecules. 2018. PMID: 30253093
-
Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering.Biomaterials. 2010 Jun;31(17):4639-56. doi: 10.1016/j.biomaterials.2010.02.044. Epub 2010 Mar 19. Biomaterials. 2010. PMID: 20303169 Free PMC article. Review.
-
Synthetic hydrogels with stiffness gradients for durotaxis study and tissue engineering scaffolds.Tissue Eng Regen Med. 2016 Apr 5;13(2):126-139. doi: 10.1007/s13770-016-0026-x. eCollection 2016 Apr. Tissue Eng Regen Med. 2016. PMID: 30603392 Free PMC article. Review.
Cited by
-
Synergistic coupling between 3D bioprinting and vascularization strategies.Biofabrication. 2023 Nov 20;16(1):012003. doi: 10.1088/1758-5090/ad0b3f. Biofabrication. 2023. PMID: 37944186 Free PMC article. Review.
-
Tuning Bulk Hydrogel Degradation by Simultaneous Control of Proteolytic Cleavage Kinetics and Hydrogel Network Architecture.ACS Macro Lett. 2018 Nov 20;7(11):1302-1307. doi: 10.1021/acsmacrolett.8b00664. Epub 2018 Oct 11. ACS Macro Lett. 2018. PMID: 32523799 Free PMC article.
-
Synthetic Capillaries to Control Microscopic Blood Flow.Sci Rep. 2016 Feb 24;6:21885. doi: 10.1038/srep21885. Sci Rep. 2016. PMID: 26905751 Free PMC article.
-
The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D.Biomaterials. 2013 Jul;34(21):5117-27. doi: 10.1016/j.biomaterials.2013.03.086. Epub 2013 Apr 19. Biomaterials. 2013. PMID: 23602364 Free PMC article.
-
A combination of matrix stiffness and degradability dictate microvascular network assembly and remodeling in cell-laden poly(ethylene glycol) hydrogels.Biomaterials. 2023 Apr;295:122050. doi: 10.1016/j.biomaterials.2023.122050. Epub 2023 Feb 15. Biomaterials. 2023. PMID: 36812843 Free PMC article.
References
-
- Smith JT, Kim DH, Reichert WM (2009) Haptotactic gradients for directed cell migration: stimulation and inhibition using soluble factors. Comb Chem High Throughput Screen 12: 598–603. - PubMed
-
- Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, et al. (2008) Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J Biol Chem 283: 13905–13912. - PubMed
-
- Mac Gabhann F, Ji JW, Popel AS (2007) VEGF gradients, receptor activation, and sprout guidance in resting and exercising skeletal muscle. J Appl Physiol 102: 722–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

