Horizontal transfer of a subtilisin gene from plants into an ancestor of the plant pathogenic fungal genus Colletotrichum
- PMID: 23554975
- PMCID: PMC3598655
- DOI: 10.1371/journal.pone.0059078
Horizontal transfer of a subtilisin gene from plants into an ancestor of the plant pathogenic fungal genus Colletotrichum
Abstract
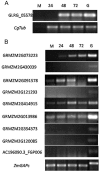
The genus Colletotrichum contains a large number of phytopathogenic fungi that produce enormous economic losses around the world. The effect of horizontal gene transfer (HGT) has not been studied yet in these organisms. Inter-Kingdom HGT into fungal genomes has been reported in the past but knowledge about the HGT between plants and fungi is particularly limited. We describe a gene in the genome of several species of the genus Colletotrichum with a strong resemblance to subtilisins typically found in plant genomes. Subtilisins are an important group of serine proteases, widely distributed in all of the kingdoms of life. Our hypothesis is that the gene was acquired by Colletotrichum spp. through (HGT) from plants to a Colletotrichum ancestor. We provide evidence to support this hypothesis in the form of phylogenetic analyses as well as a characterization of the similarity of the subtilisin at the primary, secondary and tertiary structural levels. The remarkable level of structural conservation of Colletotrichum plant-like subtilisin (CPLS) with plant subtilisins and the differences with the rest of Colletotrichum subtilisins suggests the possibility of molecular mimicry. Our phylogenetic analysis indicates that the HGT event would have occurred approximately 150-155 million years ago, after the divergence of the Colletotrichum lineage from other fungi. Gene expression analysis shows that the gene is modulated during the infection of maize by C. graminicola suggesting that it has a role in plant disease. Furthermore, the upregulation of the CPLS coincides with the downregulation of several plant genes encoding subtilisins. Based on the known roles of subtilisins in plant pathogenic fungi and the gene expression pattern that we observed, we postulate that the CPLSs have an important role in plant infection.
Conflict of interest statement
Figures




Similar articles
-
Identification of horizontally transferred genes in the genus Colletotrichum reveals a steady tempo of bacterial to fungal gene transfer.BMC Genomics. 2015 Jan 2;16(1):2. doi: 10.1186/1471-2164-16-2. BMC Genomics. 2015. PMID: 25555398 Free PMC article.
-
New insights into the evolution and structure of Colletotrichum plant-like subtilisins (CPLSs).Commun Integr Biol. 2013 Nov 1;6(6):e25727. doi: 10.4161/cib.25727. Epub 2013 Jul 16. Commun Integr Biol. 2013. PMID: 24563701 Free PMC article.
-
Extensive horizontal gene transfers between plant pathogenic fungi.BMC Biol. 2016 May 23;14:41. doi: 10.1186/s12915-016-0264-3. BMC Biol. 2016. PMID: 27215567 Free PMC article.
-
The Role of Virulence Factors in the Pathogenicity of Colletotrichum sp.Curr Protein Pept Sci. 2017;18(10):1005-1018. doi: 10.2174/1389203717666160813160727. Curr Protein Pept Sci. 2017. PMID: 27526925 Review.
-
Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range.FEMS Microbiol Rev. 2011 May;35(3):542-54. doi: 10.1111/j.1574-6976.2010.00263.x. Epub 2011 Jan 26. FEMS Microbiol Rev. 2011. PMID: 21223323 Review.
Cited by
-
Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens.PLoS Pathog. 2015 Oct 29;11(10):e1005156. doi: 10.1371/journal.ppat.1005156. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26513155 Free PMC article. No abstract available.
-
Horizontal Gene Transfer and Endophytes: An Implication for the Acquisition of Novel Traits.Plants (Basel). 2020 Mar 1;9(3):305. doi: 10.3390/plants9030305. Plants (Basel). 2020. PMID: 32121565 Free PMC article. Review.
-
Identification of horizontally transferred genes in the genus Colletotrichum reveals a steady tempo of bacterial to fungal gene transfer.BMC Genomics. 2015 Jan 2;16(1):2. doi: 10.1186/1471-2164-16-2. BMC Genomics. 2015. PMID: 25555398 Free PMC article.
-
New insights into the evolution and structure of Colletotrichum plant-like subtilisins (CPLSs).Commun Integr Biol. 2013 Nov 1;6(6):e25727. doi: 10.4161/cib.25727. Epub 2013 Jul 16. Commun Integr Biol. 2013. PMID: 24563701 Free PMC article.
-
Leucoagaricus gongylophorus uses leaf-cutting ants to vector proteolytic enzymes towards new plant substrate.ISME J. 2014 May;8(5):1032-40. doi: 10.1038/ismej.2013.231. Epub 2014 Jan 9. ISME J. 2014. PMID: 24401858 Free PMC article.
References
-
- Latunde-Dada AO (2001) Colletotrichum: tales of forcible entry, stealth, transient confinement and breakout. Mol Plant Pathol 2: 187–198 doi:10.1046/j.1464-6722.2001.00069.x - DOI - PubMed
-
- O’Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, et al. (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat Genet 44: 1060–1065 doi:10.1038/ng.2372 - DOI - PMC - PubMed
-
- Perfect SE, Green JR (2001) Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol 2: 101–108 doi:10.1046/j.1364-3703.2001.00055.x - DOI - PubMed
-
- Takano Y (2004) Molecular genetic studies on infection mechanism in Colletotrichum lagenarium . J Gen Plant Pathol 70: 390–390 doi:10.1007/s10327-004-0147-2 - DOI
-
- Dunaevsky YE, Matveeva AR, Beliakova GA, Domash VI, Belozersky MA (2007) Extracellular alkaline proteinase of Colletotrichum gloeosporioides . Biochemistry Mosc 72: 345–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

