Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids
- PMID: 23554985
- PMCID: PMC3598705
- DOI: 10.1371/journal.pone.0059145
Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids
Abstract
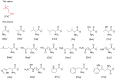
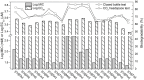
Cholinium amino acid ionic liquids ([Ch][AA] ILs), which are wholly composed of renewable biomaterials, have recently been demonstrated to have very promising properties for applications in organic synthesis and biomass pretreatment. In this work, the toxicity of these ILs toward enzymes and bacteria was assessed, and the effect of the anion on these properties is discussed. The inhibitory potentials of this type of ILs to acetylcholinesterase were weaker approximately an order of magnitude than the traditional IL 1-butyl-3-methylimidazolium tetrafluoroborate. Additionally, the [Ch][AA] ILs displayed low toxicity toward the bacteria tested. Furthermore, the biodegradability of the [Ch][AA] ILs was evaluated via the closed bottle and CO(2) headspace tests using wastewater microorganisms. All the ILs were classified as 'readily biodegradable' based on their high levels of mineralization (62-87%). The presence of extra carboxyl or amide groups on the amino acid side chain rendered the ILs significantly more susceptible to microbial breakdown. In addition, for most of the [Ch][AA] ILs, low toxicity correlated with good biodegradability. The low toxicity and high biodegradability of these novel [Ch][AA] make them promising candidates for use as environmentally friendly solvents in large-scale applications.
Conflict of interest statement
Figures
Similar articles
-
Conformation of poly(ethylene glycol) in aqueous cholinium amino acid hybrid solvents.J Colloid Interface Sci. 2021 Nov 15;602:334-343. doi: 10.1016/j.jcis.2021.06.015. Epub 2021 Jun 5. J Colloid Interface Sci. 2021. PMID: 34139531
-
Unique and generic structural features of cholinium amino acid-based biocompatible ionic liquids.Phys Chem Chem Phys. 2021 May 5;23(17):10662-10669. doi: 10.1039/d1cp00937k. Phys Chem Chem Phys. 2021. PMID: 33908525
-
(Eco)toxicity and biodegradability of selected protic and aprotic ionic liquids.J Hazard Mater. 2013 Oct 15;261:99-105. doi: 10.1016/j.jhazmat.2013.06.070. Epub 2013 Jul 6. J Hazard Mater. 2013. PMID: 23912075
-
Microstructures of Choline Amino Acid based Biocompatible Ionic Liquids.Chem Rec. 2023 Aug;23(8):e202200295. doi: 10.1002/tcr.202200295. Epub 2023 Mar 24. Chem Rec. 2023. PMID: 36960931 Review.
-
Inventory of biodegradation data of ionic liquids.Chemosphere. 2022 Jul;299:134385. doi: 10.1016/j.chemosphere.2022.134385. Epub 2022 Mar 22. Chemosphere. 2022. PMID: 35337825 Review.
Cited by
-
Assessment of the toxicity and biodegradation of amino acid-based ionic liquids.RSC Adv. 2019 Apr 1;9(18):10100-10108. doi: 10.1039/c8ra06929h. eCollection 2019 Mar 28. RSC Adv. 2019. PMID: 35520906 Free PMC article.
-
Ionic liquids as effective additives to enhance the solubility and permeation for puerarin and ferulic acid.RSC Adv. 2022 Jan 26;12(6):3416-3422. doi: 10.1039/d1ra07080k. eCollection 2022 Jan 24. RSC Adv. 2022. PMID: 35425358 Free PMC article.
-
Ionic liquids for addressing unmet needs in healthcare.Bioeng Transl Med. 2018 Jan 19;3(1):7-25. doi: 10.1002/btm2.10083. eCollection 2018 Jan. Bioeng Transl Med. 2018. PMID: 29376130 Free PMC article. Review.
-
Influence of Carboxylate Anions on Phase Behavior of Choline Ionic Liquid Mixtures.Molecules. 2020 Apr 7;25(7):1691. doi: 10.3390/molecules25071691. Molecules. 2020. PMID: 32272688 Free PMC article.
-
Choline-amino acid ionic liquids: past and recent achievements about the structure and properties of these really "green" chemicals.Biophys Rev. 2018 Jun;10(3):873-880. doi: 10.1007/s12551-018-0420-9. Epub 2018 Apr 23. Biophys Rev. 2018. PMID: 29687272 Free PMC article. Review.
References
-
- Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37: 123–150. - PubMed
-
- Mora-Pale M, Meli L, Doherty TV, Linhardt RJ, Dordick JS (2011) Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol Bioeng 108: 1229–1245. - PubMed
-
- Maia A (2011) Room temperature ionic liquids: a "green" alternative to conventional organic solvents? Mini-Rev Org Chem 8: 178–185.
-
- Pernak J, Rogoza J, Mirska I (2001) Synthesis and antimicrobial activities of new pyridinium and benzimidazolium chlorides. Eur J Med Chem 36: 313–320. - PubMed
-
- Coleman D, Gathergood N (2010) Biodegradation studies of ionic liquids. Chem Soc Rev 39: 600–637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources