Mechanisms of adhesion and subsequent actions of a haematopoietic stem cell line, HPC-7, in the injured murine intestinal microcirculation in vivo
- PMID: 23554986
- PMCID: PMC3595270
- DOI: 10.1371/journal.pone.0059150
Mechanisms of adhesion and subsequent actions of a haematopoietic stem cell line, HPC-7, in the injured murine intestinal microcirculation in vivo
Abstract
Objectives: Although haematopoietic stem cells (HSCs) migrate to injured gut, therapeutic success clinically remains poor. This has been partially attributed to limited local HSC recruitment following systemic injection. Identifying site specific adhesive mechanisms underpinning HSC-endothelial interactions may provide important information on how to enhance their recruitment and thus potentially improve therapeutic efficacy. This study determined (i) the integrins and inflammatory cyto/chemokines governing HSC adhesion to injured gut and muscle (ii) whether pre-treating HSCs with these cyto/chemokines enhanced their adhesion and (iii) whether the degree of HSC adhesion influenced their ability to modulate leukocyte recruitment.
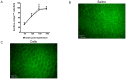
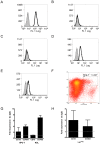
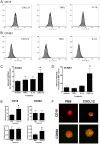
Methods: Adhesion of HPC-7, a murine HSC line, to ischaemia-reperfused (IR) injured mouse gut or cremaster muscle was monitored intravitally. Critical adhesion molecules were identified by pre-treating HPC-7 with blocking antibodies to CD18 and CD49d. To identify cyto/chemokines capable of recruiting HPC-7, adhesion was monitored following tissue exposure to TNF-α, IL-1β or CXCL12. The effects of pre-treating HPC-7 with these cyto/chemokines on surface integrin expression/clustering, adhesion to ICAM-1/VCAM-1 and recruitment in vivo was also investigated. Endogenous leukocyte adhesion following HPC-7 injection was again determined intravitally.
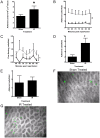
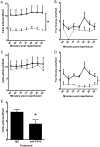
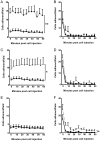
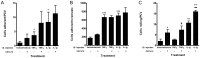
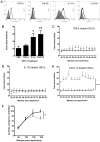
Results: IR injury increased HPC-7 adhesion in vivo, with intestinal adhesion dependent upon CD18 and muscle adhesion predominantly relying on CD49d. Only CXCL12 pre-treatment enhanced HPC-7 adhesion within injured gut, likely by increasing CD18 binding to ICAM-1 and/or CD18 surface clustering on HPC-7. Leukocyte adhesion was reduced at 4 hours post-reperfusion, but only when local HPC-7 adhesion was enhanced using CXCL12.
Conclusion: This data provides evidence that site-specific molecular mechanisms govern HPC-7 adhesion to injured tissue. Importantly, we show that HPC-7 adhesion is a modulatable event in IR injury and further demonstrate that adhesion instigated by injury alone is not sufficient for mediating anti-inflammatory effects. Enhancing local HSC presence may therefore be essential to realising their clinical potential.
Conflict of interest statement
Figures

Similar articles
-
Enhancing the adhesion of hematopoietic precursor cell integrins with hydrogen peroxide increases recruitment within murine gut.Cell Transplant. 2013;22(8):1485-99. doi: 10.3727/096368912X653192. Epub 2012 Aug 10. Cell Transplant. 2013. PMID: 22889470
-
Modulating the Adhesion of Haematopoietic Stem Cells with Chemokines to Enhance Their Recruitment to the Ischaemically Injured Murine Kidney.PLoS One. 2013 Jun 19;8(6):e66489. doi: 10.1371/journal.pone.0066489. Print 2013. PLoS One. 2013. PMID: 23840488 Free PMC article.
-
Haematopoietic stem cell recruitment to injured murine liver sinusoids depends on (alpha)4(beta)1 integrin/VCAM-1 interactions.Gut. 2010 Jan;59(1):79-87. doi: 10.1136/gut.2008.168054. Gut. 2010. PMID: 19828466
-
VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells.Leuk Lymphoma. 1997 Feb;24(5-6):423-35. doi: 10.3109/10428199709055581. Leuk Lymphoma. 1997. PMID: 9086434 Review.
-
Hematopoietic stem cell homing to injured tissues.Stem Cell Rev Rep. 2011 Sep;7(3):672-82. doi: 10.1007/s12015-011-9240-z. Stem Cell Rev Rep. 2011. PMID: 21340505 Review.
Cited by
-
Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut.Stem Cells. 2015 Sep;33(9):2785-97. doi: 10.1002/stem.2061. Epub 2015 Jun 29. Stem Cells. 2015. PMID: 26124062 Free PMC article.
-
Designing Microfluidic Devices to Sort Haematopoietic Stem Cells Based on Their Mechanical Properties.Stem Cells Int. 2019 Sep 5;2019:8540706. doi: 10.1155/2019/8540706. eCollection 2019. Stem Cells Int. 2019. PMID: 31582990 Free PMC article.
-
Tify: A quality-based frame selection tool for improving the output of unstable biomedical imaging.PLoS One. 2019 Mar 11;14(3):e0213162. doi: 10.1371/journal.pone.0213162. eCollection 2019. PLoS One. 2019. PMID: 30856207 Free PMC article.
-
Live Intravital Imaging of Cellular Trafficking in the Cardiac Microvasculature-Beating the Odds.Front Immunol. 2019 Nov 26;10:2782. doi: 10.3389/fimmu.2019.02782. eCollection 2019. Front Immunol. 2019. PMID: 31849965 Free PMC article. Review.
-
Mesenchymal Stem Cell Deformability and Implications for Microvascular Sequestration.Ann Biomed Eng. 2018 Apr;46(4):640-654. doi: 10.1007/s10439-018-1985-y. Epub 2018 Jan 19. Ann Biomed Eng. 2018. PMID: 29352448 Free PMC article.
References
-
- Garcia-Gomez I, Elvira G, Zapata AG, Lamana ML, Ramirez M, et al. (2010) Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther 10: 1453–1468. - PubMed
-
- Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, et al. (2005) Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology 128: 552–563. - PubMed
-
- Schwarting S, Litwak S, Hao W, Bahr M, Weise J, et al. (2008) Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 39: 2867–2875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

