Alteration of the thymic T cell repertoire by rotavirus infection is associated with delayed type 1 diabetes development in non-obese diabetic mice
- PMID: 23554993
- PMCID: PMC3598695
- DOI: 10.1371/journal.pone.0059182
Alteration of the thymic T cell repertoire by rotavirus infection is associated with delayed type 1 diabetes development in non-obese diabetic mice
Abstract
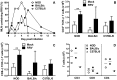
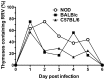
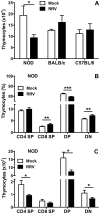
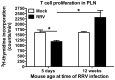
Rotaviruses are implicated as a viral trigger for the acceleration of type 1 diabetes in children. Infection of adult non-obese diabetic (NOD) mice with rotavirus strain RRV accelerates diabetes development, whereas RRV infection in infant NOD mice delays diabetes onset. In this study of infant mice, RRV titers and lymphocyte populations in the intestine, mesenteric lymph nodes (MLN) and thymus of NOD mice were compared with those in diabetes-resistant BALB/c and C57BL/6 mice. Enhanced intestinal RRV infection occurred in NOD mice compared with the other mouse strains. This was associated with increases in the frequency of CD8αβ TCRαβ intraepithelial lymphocytes, and their PD-L1 expression. Virus spread to the MLN and T cell numbers there also were greatest in NOD mice. Thymic RRV infection is shown here in all mouse strains, often in combination with alterations in T cell ontogeny. Infection lowered thymocyte numbers in infant NOD and C57BL/6 mice, whereas thymocyte production was unaltered overall in infant BALB/c mice. In the NOD mouse thymus, effector CD4(+) T cell numbers were reduced by infection, whereas regulatory T cell numbers were maintained. It is proposed that maintenance of thymic regulatory T cell numbers may contribute to the increased suppression of inflammatory T cells in response to a strong stimulus observed in pancreatic lymph nodes of adult mice infected as infants. These findings show that rotavirus replication is enhanced in diabetes-prone mice, and provide evidence that thymic T cell alterations may contribute to the delayed diabetes onset following RRV infection.
Conflict of interest statement
Figures



References
-
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, et al. (2006) Rotavirus vaccines: current prospects and future challenges. Lancet 368: 323–332. - PubMed
-
- Maclaren N, Lan M, Coutant R, Schatz D, Silverstein J, et al. (1999) Only multiple autoantibodies to islet cells (ICA), insulin, GAD65, IA-2 and IA-2beta predict immune-mediated (Type 1) diabetes in relatives. J Autoimmun 12: 279–287. - PubMed
-
- Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, et al. (2004) Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 89: 3896–3902. - PubMed
-
- Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, et al. (2000) Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49: 1319–1324. - PubMed
-
- Lehuen A, Diana J, Zaccone P, Cooke A (2010) Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 10: 501–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

