γ-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after β-catenin knockdown
- PMID: 23555187
- PMCID: PMC3612914
- DOI: 10.1593/neo.122098
γ-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after β-catenin knockdown
Abstract
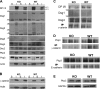
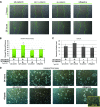
β-Catenin is important in liver homeostasis as a part of Wnt signaling and adherens junctions (AJs), while its aberrant activation is observed in hepatocellular carcinoma (HCC). We have reported hepatocyte-specific β-catenin knockout (KO) mice to lack adhesive defects as γ-catenin compensated at AJ. Because γ-catenin is a desmosomal protein, we asked if its increase in KO might deregulate desmosomes. No changes in desmosomal proteins or ultrastructure other than increased plakophilin-3 were observed. To further elucidate the role and regulation of γ-catenin, we contemplate an in vitro model and show γ-catenin increase in HCC cells upon β-catenin knockdown (KD). Here, γ-catenin is unable to rescue β-catenin/T cell factor (TCF) reporter activity; however, it sufficiently compensates at AJs as assessed by scratch wound assay, centrifugal assay for cell adhesion (CAFCA), and hanging drop assays. γ-Catenin increase is observed only after β-catenin protein decrease and not after blockade of its transactivation. γ-Catenin increase is associated with enhanced serine/threonine phosphorylation and abrogated by protein kinase A (PKA) inhibition. In fact, several PKA-binding sites were detected in γ-catenin by in silico analysis. Intriguingly γ-catenin KD led to increased β-catenin levels and transactivation. Thus, γ-catenin compensates for β-catenin loss at AJ without affecting desmosomes but is unable to fulfill functions in Wnt signaling. γ-Catenin stabilization after β-catenin loss is brought about by PKA. Catenin-sensing mechanism may depend on absolute β-catenin levels and not its activity. Anti-β-catenin therapies for HCC affecting total β-catenin may target aberrant Wnt signaling without negatively impacting intercellular adhesion, provided mechanisms leading to γ-catenin stabilization are spared.
Figures




References
-
- El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Legoix P, Bluteau O, Bayer J, Perret C, Balabaud C, Belghiti J, Franco D, Thomas G, Laurent-Puig P, Zucman-Rossi J. Beta-catenin mutations in hepatocellular carcinoma correlate with a low rate of loss of heterozygosity. Oncogene. 1999;18:4044–4046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
