Origin and evolution of protein fold designs inferred from phylogenomic analysis of CATH domain structures in proteomes
- PMID: 23555236
- PMCID: PMC3610613
- DOI: 10.1371/journal.pcbi.1003009
Origin and evolution of protein fold designs inferred from phylogenomic analysis of CATH domain structures in proteomes
Abstract
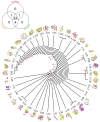
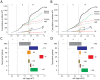
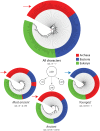
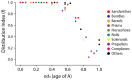
The spatial arrangements of secondary structures in proteins, irrespective of their connectivity, depict the overall shape and organization of protein domains. These features have been used in the CATH and SCOP classifications to hierarchically partition fold space and define the architectural make up of proteins. Here we use phylogenomic methods and a census of CATH structures in hundreds of genomes to study the origin and diversification of protein architectures (A) and their associated topologies (T) and superfamilies (H). Phylogenies that describe the evolution of domain structures and proteomes were reconstructed from the structural census and used to generate timelines of domain discovery. Phylogenies of CATH domains at T and H levels of structural abstraction and associated chronologies revealed patterns of reductive evolution, the early rise of Archaea, three epochs in the evolution of the protein world, and patterns of structural sharing between superkingdoms. Phylogenies of proteomes confirmed the early appearance of Archaea. While these findings are in agreement with previous phylogenomic studies based on the SCOP classification, phylogenies unveiled sharing patterns between Archaea and Eukarya that are recent and can explain the canonical bacterial rooting typically recovered from sequence analysis. Phylogenies of CATH domains at A level uncovered general patterns of architectural origin and diversification. The tree of A structures showed that ancient structural designs such as the 3-layer (αβα) sandwich (3.40) or the orthogonal bundle (1.10) are comparatively simpler in their makeup and are involved in basic cellular functions. In contrast, modern structural designs such as prisms, propellers, 2-solenoid, super-roll, clam, trefoil and box are not widely distributed and were probably adopted to perform specialized functions. Our timelines therefore uncover a universal tendency towards protein structural complexity that is remarkable.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Caetano-Anolles G, Wang M, Caetano-Anolles D, Mittenthal JE (2009) The origin, evolution and structure of the protein world. Biochem J 417: 621–637. - PubMed
-
- Andreeva A, Murzin AG (2006) Evolution of protein fold in the presence of functional constraints. Curr Opin Struct Biol 16: 399–408. - PubMed
-
- Worth CL, Gong S, Blundell TL (2009) Structural and functional constraints in the evolution of protein families. Nat Rev Mol Cell Biol 10: 709–720. - PubMed
-
- Murzin AG, Brenner SE, Hubbard T, Chothia C (1995) SCOP: A structural classification of proteins database for the investigation of sequences and structures. J Mol Biol 247: 536–540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

