Temporal analysis of hepatitis C virus cell entry with occludin directed blocking antibodies
- PMID: 23555257
- PMCID: PMC3605286
- DOI: 10.1371/journal.ppat.1003244
Temporal analysis of hepatitis C virus cell entry with occludin directed blocking antibodies
Abstract
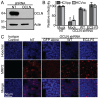
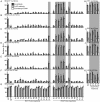
Hepatitis C virus (HCV) is a major cause of liver disease worldwide. A better understanding of its life cycle, including the process of host cell entry, is important for the development of HCV therapies and model systems. Based on the requirement for numerous host factors, including the two tight junction proteins claudin-1 (CLDN1) and occludin (OCLN), HCV cell entry has been proposed to be a multi-step process. The lack of OCLN-specific inhibitors has prevented a comprehensive analysis of this process. To study the role of OCLN in HCV cell entry, we created OCLN mutants whose HCV cell entry activities could be inhibited by antibodies. These mutants were expressed in polarized HepG2 cells engineered to support the complete HCV life cycle by CD81 and miR-122 expression and synchronized infection assays were performed to define the kinetics of HCV cell entry. During these studies, OCLN utilization differences between HCV isolates were observed, supporting a model that HCV directly interacts with OCLN. In HepG2 cells, both HCV cell entry and tight junction formation were impaired by OCLN silencing and restored by expression of antibody regulatable OCLN mutant. Synchronized infection assays showed that glycosaminoglycans and SR-BI mediated host cell binding, while CD81, CLDN1 and OCLN all acted sequentially at a post-binding stage prior to endosomal acidification. These results fit a model where the tight junction region is the last to be encountered by the virion prior to internalization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Hepatitis C Virus Entry: Protein Interactions and Fusion Determinants Governing Productive Hepatocyte Invasion.Cold Spring Harb Perspect Med. 2020 Feb 3;10(2):a036830. doi: 10.1101/cshperspect.a036830. Cold Spring Harb Perspect Med. 2020. PMID: 31427285 Free PMC article. Review.
-
Monoclonal Antibodies against Occludin Completely Prevented Hepatitis C Virus Infection in a Mouse Model.J Virol. 2018 Mar 28;92(8):e02258-17. doi: 10.1128/JVI.02258-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29437969 Free PMC article.
-
Mice Expressing Minimally Humanized CD81 and Occludin Genes Support Hepatitis C Virus Uptake In Vivo.J Virol. 2017 Jan 31;91(4):e01799-16. doi: 10.1128/JVI.01799-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928007 Free PMC article.
-
Infection with hepatitis C virus depends on TACSTD2, a regulator of claudin-1 and occludin highly downregulated in hepatocellular carcinoma.PLoS Pathog. 2018 Mar 14;14(3):e1006916. doi: 10.1371/journal.ppat.1006916. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29538454 Free PMC article.
-
Hepatitis C virus infection and tight junction proteins: The ties that bind.Biochim Biophys Acta Biomembr. 2020 Jul 1;1862(7):183296. doi: 10.1016/j.bbamem.2020.183296. Epub 2020 Apr 5. Biochim Biophys Acta Biomembr. 2020. PMID: 32268133 Free PMC article. Review.
Cited by
-
Selection of a hepatitis C virus with altered entry factor requirements reveals a genetic interaction between the E1 glycoprotein and claudins.Hepatology. 2015 Oct;62(4):1059-69. doi: 10.1002/hep.27815. Epub 2015 May 12. Hepatology. 2015. PMID: 25820616 Free PMC article.
-
Hepatitis C Virus Entry: Protein Interactions and Fusion Determinants Governing Productive Hepatocyte Invasion.Cold Spring Harb Perspect Med. 2020 Feb 3;10(2):a036830. doi: 10.1101/cshperspect.a036830. Cold Spring Harb Perspect Med. 2020. PMID: 31427285 Free PMC article. Review.
-
Hepatitis C Virus Entry: An Intriguingly Complex and Highly Regulated Process.Int J Mol Sci. 2020 Mar 18;21(6):2091. doi: 10.3390/ijms21062091. Int J Mol Sci. 2020. PMID: 32197477 Free PMC article. Review.
-
The Humoral Immune Response to HCV: Understanding is Key to Vaccine Development.Front Immunol. 2014 Nov 10;5:550. doi: 10.3389/fimmu.2014.00550. eCollection 2014. Front Immunol. 2014. PMID: 25426115 Free PMC article. Review.
-
HepG2 cells mount an effective antiviral interferon-lambda based innate immune response to hepatitis C virus infection.Hepatology. 2014 Oct;60(4):1170-9. doi: 10.1002/hep.27227. Epub 2014 Aug 21. Hepatology. 2014. PMID: 24833036 Free PMC article.
References
-
- Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, et al. (1999) The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 341: 556–562. - PubMed
-
- Montalto G, Cervello M, Giannitrapani L, Dantona F, Terranova A, et al. (2002) Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci 963: 13–20. - PubMed
-
- Brown RS Jr (2005) Hepatitis C and liver transplantation. Nature 436: 973–978. - PubMed
-
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, et al. (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364: 2405–2416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56AI091792/AI/NIAID NIH HHS/United States
- R01 DK095125/DK/NIDDK NIH HHS/United States
- 5R24 CA095823-04/CA/NCI NIH HHS/United States
- F30DK096892/DK/NIDDK NIH HHS/United States
- AI07647/AI/NIAID NIH HHS/United States
- 1 S10 RR0 9145-01/RR/NCRR NIH HHS/United States
- F30 DK096892/DK/NIDDK NIH HHS/United States
- R24 CA095823/CA/NCI NIH HHS/United States
- R00AI077800/AI/NIAID NIH HHS/United States
- T32 AI007647/AI/NIAID NIH HHS/United States
- R00 AI077800/AI/NIAID NIH HHS/United States
- R56 AI091792/AI/NIAID NIH HHS/United States
- R01DK095125/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

