Natalizumab treatment reduces fatigue in multiple sclerosis. Results from the TYNERGY trial; a study in the real life setting
- PMID: 23555589
- PMCID: PMC3605436
- DOI: 10.1371/journal.pone.0058643
Natalizumab treatment reduces fatigue in multiple sclerosis. Results from the TYNERGY trial; a study in the real life setting
Abstract
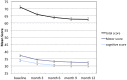
Fatigue is a significant symptom in multiple sclerosis (MS) patients. First-generation disease modifying therapies (DMTs) are at best moderately effective to improve fatigue. Observations from small cohorts have indicated that natalizumab, an antibody targeting VLA-4, may reduce MS-related fatigue. The TYNERGY study aimed to further evaluate the effects of natalizumab treatment on MS-related fatigue. In this one-armed clinical trial including 195 MS patients, natalizumab was prescribed in a real-life setting, and a validated questionnaire, the Fatigue Scale for Motor and Cognitive functions (FSMC), was used both before and after 12 months of treatment to evaluate a possible change in the fatigue experienced by the patients. In the treated cohort all measured variables, that is, fatigue score, quality of life, sleepiness, depression, cognition, and disability progression were improved from baseline (all p values<0.0001). Walking speed as measured by the six-minute walk-test also increased at month 12 (p = 0.0016). All patients were aware of the nature of the treatment agent, and of the study outcomes.
Conclusion: Natalizumab, as used in a real-life setting, might improve MS-related fatigue based on the results from this one-armed un-controlled stud. Also other parameters related to patients' quality of life seemed to improve with natalizumab treatment.
Trial registration: ClinicalTrials.gov NCT00884481.
Conflict of interest statement
Figures
References
-
- Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ (1994) The impact of fatigue on patients with multiple sclerosis. Canadian Journal of Neurological Sciences 21: 9–14. - PubMed
-
- Wood B, van der Mei I, Ponsonby AL, Pittas F, Quinn S, et al. (2012) Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Multiple sclerosis 19 2: 217–224. - PubMed
-
- Amato MP, Portaccio E (2012) Management options in multiple sclerosis-associated fatigue. Expert opinion on pharmacotherapy 13: 207–216. - PubMed
-
- Comi G, Leocani L, Rossi P, Colombo B (2001) Physiopathology and treatment of fatigue in multiple sclerosis. Journal of Neurology 248: 174–179. - PubMed
-
- Whitaker J, Mitchell G (1997) Clinical features of multiple sclerosis. In: Raine CS, McFarland HF, Tourtellotte WW, editors. Multiple Sclerosis Clinical and Pathogenetic Basis. London: Chapman and Hall Medical. pp. 3–19.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

