Silencing of RASSF3 by DNA hypermethylation is associated with tumorigenesis in somatotroph adenomas
- PMID: 23555615
- PMCID: PMC3610897
- DOI: 10.1371/journal.pone.0059024
Silencing of RASSF3 by DNA hypermethylation is associated with tumorigenesis in somatotroph adenomas
Abstract
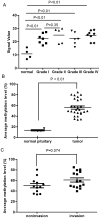
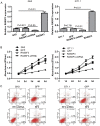
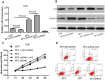
The pathogenic mechanisms underlying pituitary somatotroph adenoma formation, progression are poorly understood. To identify candidate tumor suppressor genes involved in pituitary somatotroph adenoma tumorigenesis, we used HG18 CpG plus Promoter Microarray in 27 human somatotroph adenomas and 4 normal human adenohypophyses. RASSF3 was found with frequent methylation of CpG island in its promoter region in somatotroph adenomas but rarely in adenohypophyses. This result was confirmed by pyrosequencing analysis. We also found that RASSF3 mRNA level correlated negatively to its gene promoter methylation level. RASSF3 hypermethylation and downregulation was also observed in rat GH3 and mouse GT1.1 somatotroph adenoma cell lines. 5-Aza-2' deoxycytidine and trichostatin-A treatment induced RASSF3 promoter demethylation, and restored its expression in GH3 and GT1.1 cell lines. RASSF3 overexpression in GH3 and GT1.1 cells inhibited proliferation, induced apoptosis accompanied by increased Bax, p53, and caspase-3 protein and decreased Bcl-2 protein expression. We also found that the antitumor effect of RASSF3 was p53 dependent, and p53 knockdown blocked RASSF3-induced apoptosis and growth inhibition. Taken together, our results suggest that hypermethylation-induced RASSF3 silencing plays an important role in the tumorigenesis of pituitary somatotroph adenomas.
Conflict of interest statement
Figures




Similar articles
-
Silencing of HEPN1 is responsible for the aggressive biological behavior of pituitary somatotroph adenomas.Cell Physiol Biochem. 2013;31(2-3):379-88. doi: 10.1159/000343375. Epub 2013 Mar 8. Cell Physiol Biochem. 2013. Retraction in: Cell Physiol Biochem. 2022 Dec 28;56(6):744. doi: 10.33594/000000597. PMID: 23548416 Retracted.
-
STAT3 upregulation in pituitary somatotroph adenomas induces growth hormone hypersecretion.J Clin Invest. 2015 Apr;125(4):1692-702. doi: 10.1172/JCI78173. Epub 2015 Mar 16. J Clin Invest. 2015. PMID: 25774503 Free PMC article.
-
Aberrant methylation and silencing of ARHI, an imprinted tumor suppressor gene in which the function is lost in breast cancers.Cancer Res. 2003 Jul 15;63(14):4174-80. Cancer Res. 2003. PMID: 12874023
-
The absence of PRDM2 involved the tumorigenesis of somatotroph adenomas through regulating c-Myc.Gene. 2020 May 5;737:144456. doi: 10.1016/j.gene.2020.144456. Epub 2020 Feb 7. Gene. 2020. PMID: 32044406
-
RASSF3 downregulation increases malignant phenotypes of non-small cell lung cancer.Lung Cancer. 2014 Jan;83(1):23-9. doi: 10.1016/j.lungcan.2013.10.014. Epub 2013 Oct 31. Lung Cancer. 2014. PMID: 24246507
Cited by
-
Animal models of pituitary neoplasia.Mol Cell Endocrinol. 2016 Feb 5;421:68-81. doi: 10.1016/j.mce.2015.08.024. Epub 2015 Aug 28. Mol Cell Endocrinol. 2016. PMID: 26320859 Free PMC article. Review.
-
Pumping the brakes on RAS - negative regulators and death effectors of RAS.J Cell Sci. 2020 Feb 10;133(3):jcs238865. doi: 10.1242/jcs.238865. J Cell Sci. 2020. PMID: 32041893 Free PMC article. Review.
-
The role of genetic and epigenetic changes in pituitary tumorigenesis.Neurol Med Chir (Tokyo). 2014;54(12):943-57. doi: 10.2176/nmc.ra.2014-0184. Epub 2014 Nov 29. Neurol Med Chir (Tokyo). 2014. PMID: 25446387 Free PMC article. Review.
-
The Role and Function of Ras-association domain family in Cancer: A Review.Genes Dis. 2019 Jul 27;6(4):378-384. doi: 10.1016/j.gendis.2019.07.008. eCollection 2019 Dec. Genes Dis. 2019. PMID: 31832517 Free PMC article. Review.
-
Genetic and epigenetic mutations of tumor suppressive genes in sporadic pituitary adenoma.Mol Cell Endocrinol. 2014 Apr 5;386(1-2):16-33. doi: 10.1016/j.mce.2013.09.006. Epub 2013 Sep 11. Mol Cell Endocrinol. 2014. PMID: 24035864 Free PMC article. Review.
References
-
- Asa SL, Ezzat S (2009) The pathogenesis of pituitary tumors. Annu Rev Pathol 4: 97–126. - PubMed
-
- Asa SL, Ezzat S (1998) The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev 19: 798–827. - PubMed
-
- Asa SL, Ezzat S (2002) The pathogenesis of pituitary tumours. Nat Rev Cancer 2: 836–849. - PubMed
-
- Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7: 257–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

