Frequent engagement of RelB activation is critical for cell survival in multiple myeloma
- PMID: 23555623
- PMCID: PMC3610937
- DOI: 10.1371/journal.pone.0059127
Frequent engagement of RelB activation is critical for cell survival in multiple myeloma
Abstract
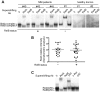
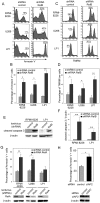
The NF-κB family of transcription factors has emerged as a key player in the pathogenesis of multiple myeloma (MM). NF-κB is activated by at least two major signaling pathways. The classical pathway results in the activation of mainly RelA containing dimers, whereas the alternative pathway leads to the activation of RelB/p52 and RelB/p50 heterodimers. Activating mutations in regulators of the alternative pathway have been identified in 17% of MM patients. However, the status of RelB activation per se and its role in the regulation of cell survival in MM has not been investigated. Here, we reveal that 40% of newly diagnosed MM patients have a constitutive RelB DNA-binding activity in CD138(+) tumor cells, and we show an association with increased expression of a subset of anti-apoptotic NF-κB target genes, such as cIAP2. Furthermore, we demonstrate that RelB exerts a crucial anti-apoptotic activity in MM cells. Our findings indicate that RelB activation is key for promoting MM cell survival through the upregulation of anti-apoptotic proteins. Altogether, our study provides the framework for the development of new molecules targeting RelB in the treatment of MM.
Conflict of interest statement
Figures


Similar articles
-
Non-canonical NFκB mutations reinforce pro-survival TNF response in multiple myeloma through an autoregulatory RelB:p50 NFκB pathway.Oncogene. 2017 Mar;36(10):1417-1429. doi: 10.1038/onc.2016.309. Epub 2016 Sep 19. Oncogene. 2017. PMID: 27641334 Free PMC article.
-
Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma.Cancer Cell. 2007 Aug;12(2):115-30. doi: 10.1016/j.ccr.2007.07.004. Cancer Cell. 2007. PMID: 17692804 Free PMC article.
-
The alternative RelB NF-κB subunit is a novel critical player in diffuse large B-cell lymphoma.Blood. 2022 Jan 20;139(3):384-398. doi: 10.1182/blood.2020010039. Blood. 2022. PMID: 34232979
-
NF-κB pathways in hematological malignancies.Cell Mol Life Sci. 2014 Jun;71(11):2083-102. doi: 10.1007/s00018-013-1545-4. Epub 2014 Jan 14. Cell Mol Life Sci. 2014. PMID: 24419302 Free PMC article. Review.
-
The noncanonical NFκB pathway: Regulatory mechanisms in health and disease.WIREs Mech Dis. 2024 Nov-Dec;16(6):e1646. doi: 10.1002/wsbm.1646. Epub 2024 Apr 18. WIREs Mech Dis. 2024. PMID: 38634218 Review.
Cited by
-
Extracellular acidification stimulates GPR68 mediated IL-8 production in human pancreatic β cells.Sci Rep. 2016 May 11;6:25765. doi: 10.1038/srep25765. Sci Rep. 2016. PMID: 27166427 Free PMC article.
-
Prognostic value of expression of nuclear factor kappa-B/p65 in non-GCB DLBCL patients.Oncotarget. 2017 Feb 7;8(6):9708-9716. doi: 10.18632/oncotarget.14182. Oncotarget. 2017. PMID: 28039454 Free PMC article.
-
RELB: A novel prognostic marker for glioblastoma as identified by population-based analysis.Oncol Lett. 2019 Jul;18(1):386-394. doi: 10.3892/ol.2019.10296. Epub 2019 Apr 30. Oncol Lett. 2019. PMID: 31289510 Free PMC article.
-
The NF-κB Activating Pathways in Multiple Myeloma.Biomedicines. 2018 May 16;6(2):59. doi: 10.3390/biomedicines6020059. Biomedicines. 2018. PMID: 29772694 Free PMC article. Review.
-
NF-κB signaling activation and roles in thyroid cancers: implication of MAP3K14/NIK.Oncogenesis. 2023 Nov 16;12(1):55. doi: 10.1038/s41389-023-00496-w. Oncogenesis. 2023. PMID: 37973791 Free PMC article.
References
-
- Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364: 1046–1060. - PubMed
-
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC (2007) Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 7: 585–598. - PubMed
-
- Laubach J, Richardson P, Anderson K (2011) Multiple myeloma. Annu Rev Med 62: 249–264. - PubMed
-
- Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, et al. (2012) Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol 9: 135–143. - PubMed
-
- Hideshima T, Anderson KC (2002) Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer 2: 927–937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

