Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression
- PMID: 23555642
- PMCID: PMC3608626
- DOI: 10.1371/journal.pone.0059259
Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression
Abstract
Background: We previously showed that evaluation of anti-inflammatory activities of lactic acid bacteria in porcine intestinal epithelial (PIE) cells is useful for selecting potentially immunobiotic strains.
Objective: The aims of the present study were: i) to select potentially immunomodulatory bifidobacteria that beneficially modulate the Toll-like receptor (TLR)-4-triggered inflammatory response in PIE cells and; ii) to gain insight into the molecular mechanisms involved in the anti-inflammatory effect of immunobiotics by evaluating the role of TLR2 and TLR negative regulators in the modulation of proinflammatory cytokine production and activation of mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways in PIE cells.
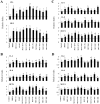
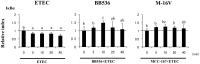
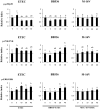
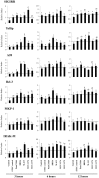
Results: Bifidobacteria longum BB536 and B. breve M-16V strains significantly downregulated levels of interleukin (IL)-8, monocyte chemotactic protein (MCP)-1 and IL-6 in PIE cells challenged with heat-killed enterotoxigenic Escherichia coli. Moreover, BB536 and M-16V strains attenuated the proinflammatory response by modulating the NF-κB and MAPK pathways. In addition, our findings provide evidence for a key role for the ubiquitin-editing enzyme A20 in the anti-inflammatory effect of immunobiotic bifidobacteria in PIE cells.
Conclusions: We show new data regarding the mechanism involved in the anti-inflammatory effect of immunobiotics. Several strains with immunoregulatory capabilities used a common mechanism to induce tolerance in PIE cells. Immunoregulatory strains interacted with TLR2, upregulated the expression of A20 in PIE cells, and beneficially modulated the subsequent TLR4 activation by reducing the activation of MAPK and NF-κB pathways and the production of proinflammatory cytokines. We also show that the combination of TLR2 activation and A20 induction can be used as biomarkers to screen and select potential immunoregulatory bifidobacteria strains.
Conflict of interest statement
Figures


References
-
- Westendorf AM, Fleissner D, Hansen W, Buer J (2010) T cells, dendritic cells and epithelial cells in intestinal homeostasis. Int J Med Microbiol 300: 11–18. - PubMed
-
- Cerf-Bensussan N, Gaboriau-Routhiau V (2010) The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 10: 735–744. - PubMed
-
- Villena J, Aso H, Alvarez S, Kitazawa H (2012) Porcine toll-like receptors and their crosstalk with immunobiotics: impact in the regulation of gut inflammatory immunity. In: Probiotics: Sources, Types and Health Benefits. NOVA Science publishers, In press.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

