Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review
- PMID: 23555704
- PMCID: PMC3608726
- DOI: 10.1371/journal.pone.0059551
Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review
Abstract
Background: Recent studies suggest certain antiretroviral therapy (ART) drugs are associated with increases in cardiovascular disease.
Purpose: We performed a systematic review and meta-analysis to summarize the available evidence, with the goal of elucidating whether specific ART drugs are associated with an increased risk of myocardial infarction (MI).
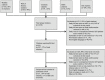
Data sources: We searched Medline, Web of Science, the Cochrane Library, and abstract archives from the Conference on Retroviruses and Opportunistic Infections and International AIDS Society up to June 2011 to identify published articles and abstracts.
Study selection: Eligible studies were comparative and included MI, strokes, or other cardiovascular events as outcomes.
Data extraction: Eligibility screening, data extraction, and quality assessment were performed independently by two investigators.
Data synthesis: Random effects methods and Fisher's combined probability test were used to summarize evidence.
Findings: Twenty-seven studies met inclusion criteria, with 8 contributing to a formal meta-analysis. Findings based on two observational studies indicated an increase in risk of MI for patients recently exposed (usually defined as within last 6 months) to abacavir (RR 1.92, 95% CI 1.51-2.42) and protease inhibitors (PI) (RR 2.13, 95% CI 1.06-4.28). Our analysis also suggested an increased risk associated with each additional year of exposure to indinavir (RR 1.11, 95% CI 1.05-1.17) and lopinavir (RR 1.22, 95% CI 1.01-1.47). Our findings of increased cardiovascular risk from abacavir and PIs were in contrast to four published meta-analyses based on secondary analyses of randomized controlled trials, which found no increased risk from cardiovascular disease.
Conclusion: Although observational studies implicated specific drugs, the evidence is mixed. Further, meta-analyses of randomized trials did not find increased risk from abacavir and PIs. Our findings that implicate specific ARTs in the observational setting provide sufficient evidence to warrant further investigation of this relationship in studies designed for that purpose.
Conflict of interest statement
Figures



References
-
- Grinspoon S, Carr A (2005) Cardiovascular Risk and Body-Fat Abnormalities in HIV-Infected Adults. N Engl J Med 352: 48–62. - PubMed
-
- Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, et al. (2003) Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 349: 1993–2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

